Abstract
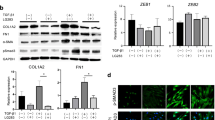
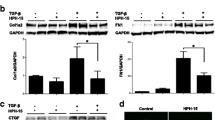
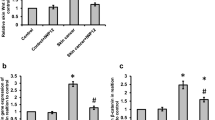
Skin fibrosis is one of the major features of scleroderma. WNT/β-catenin signaling is associated with the progression of skin fibrosis. In this study, we aimed to determine the effect of icaritin (IT), a natural compound, on scleroderma-related skin fibrosis and its mechanisms. We found that IT could reduce the expression of COL1A1, COL1A2, COL3A1, CTGF, and α-SMA in human foreskin fibroblasts (HFF-1 cells), scleroderma skin fibroblasts (SSF cells), and TGF-β-induced HFF-1 cells. Wnt/β-catenin signaling was shown to be suppressed by IT. Additionally, IT activated AMPK signaling in HFF-1 cells. In conclusion, IT has an anti-skin fibrotic effect through activation of AMPK signaling and inhibition of WNT/β-catenin signaling. Our findings indicate the potential role of IT in the treatment of scleroderma and provide novel insight for the selection of drug therapy for scleroderma.




Similar content being viewed by others
References
Ferreli, C., Gasparini, G., & Parodi, A., et al. (2017). Cutaneous manifestations of scleroderma and scleroderma-like disorders: a comprehensive review. Clinical Reviews in Allergy and Immunology, 53, 306–336.
Volkmann, E. R., & Varga, J. (2019). Emerging targets of disease-modifying therapy for systemic sclerosis. Nature Reviews Rheumatology, 15, 208–224.
Korman, B. (2019). Evolving insights into the cellular and molecular pathogenesis of fibrosis in systemic sclerosis. Translational Research, 209, 77–89.
Garrett, S. M., Baker Frost, D., & Feghali-Bostwick, C. (2017). The mighty fibroblast and its utility in scleroderma research. Journal of Scleroderma Related Disorders, 2, 69–134.
Daoussis, D., & Liossis, S.-N. (2019). Treatment of systemic sclerosis associated fibrotic manifestations: current options and future directions. Mediterranean Journal of Rheumatology, 30, 33–37.
Wang, Q., Zang, W., & Li, H., et al. (2018). Wenyang Huazhuo Tongluo formula inhibits fibrosis via suppressing Wnt/β-catenin signaling pathway in a Bleomycin-induced systemic sclerosis mouse model. Chinese Medicine, 13, 17–17.
Liu, Q., Lu, J., & Lin, J., et al. (2019). Salvianolic acid B attenuates experimental skin fibrosis of systemic sclerosis. Biomedicine and Pharmacotherapy, 110, 546–553.
Wu, T., Chu, H., & Tu, W., et al. (2014). Dissection of the mechanism of traditional Chinese medical prescription-Yiqihuoxue formula as an effective anti-fibrotic treatment for systemic sclerosis. BMC Complementary and Alternative Medicine, 14, 224–224.
Yan, X.-n., Feng, J., Li, W.-b., Cui, R., & Shi, B.-j. (2007). Effects of Wenyang Chubi Decoction on connective tissue growth factor and collagen-I in a mouse model of scleroderma. Zhong Xi Yi Jie He Xue Bao, 5, 526–530.
Sze, S. C., Tong, Y., Ng, T. B., Cheng, C. L., & Cheung, H. P. (2010). Herba Epimedii: anti-oxidative properties and its medical implications. Molecules, 15, 7861–7870.
Chen, Y., Zhao, Y. H., Jia, X. B., & Hu, M. (2008). Intestinal absorption mechanisms of prenylated flavonoids present in the heat-processed Epimedium koreanum Nakai (Yin Yanghuo). Pharmaceutical Research, 25, 2190–2199.
Wu, H., Lien, E. J., & Lien, L. L. (2003). Chemical and pharmacological investigations of Epimedium species: a survey. Progress in Drug Research, 60, 1–57.
Li, J., Liu, P., & Zhang, R., et al. (2011). Icaritin induces cell death in activated hepatic stellate cells through mitochondrial activated apoptosis and ameliorates the development of liver fibrosis in rats. Journal of Ethnopharmacology, 137, 714–723.
Chor, S. Y., Hui, A. Y., & To, K. F., et al. (2005). Anti-proliferative and pro-apoptotic effects of herbal medicine on hepatic stellate cell. Journal of Ethnopharmacology, 100, 180–186.
Li, Z., Zhou, L., & Wang, Y., et al. (2017). (Pro)renin Receptor Is an Amplifier of Wnt/beta-Catenin Signaling in Kidney Injury and Fibrosis. Journal of the American Society of Nephrology, 28, 2393–2408.
Blyszczuk, P., Müller-Edenborn, B., & Valenta, T., et al. (2017). Transforming growth factor-beta-dependent Wnt secretion controls myofibroblast formation and myocardial fibrosis progression in experimental autoimmune myocarditis. European Heart Journal, 38, 1413–1425.
Lam, A. P., Herazo-Maya, J. D., & Sennello, J. A., et al. (2014). Wnt coreceptor Lrp5 is a driver of idiopathic pulmonary fibrosis. American Journal of Respiratory and Critical Care Medicine, 190, 185–195.
Guo, Y., Xiao, L., Sun, L., & Liu, F. (2012). Wnt/beta-catenin signaling: a promising new target for fibrosis diseases. Physiological Research, 61, 337–346.
Beyer, C., Reichert, H., & Akan, H., et al. (2013). Blockade of canonical Wnt signalling ameliorates experimental dermal fibrosis. Annals of the Rheumatic Diseases, 72, 1255–1258.
Wei, J., Fang, F., & Lam, A. P., et al. (2012). Wnt/beta-catenin signaling is hyperactivated in systemic sclerosis and induces Smad-dependent fibrotic responses in mesenchymal cells. Arthritis and Rheumatology, 64, 2734–2745.
Beyer, C., Schramm, A., & Akhmetshina, A., et al. (2012). beta-catenin is a central mediator of pro-fibrotic Wnt signaling in systemic sclerosis. Annals of the Rheumatic Diseases, 71, 761–767.
Garcia, D., & Shaw, R. J. (2017). AMPK: mechanisms of cellular energy sensing and restoration of metabolic balance. Molecular Cell, 66, 789–800.
Zhang, P., Song, Y., & Sun, Y., et al. (2018). AMPK/GSK3beta/beta-catenin cascade-triggered overexpression of CEMIP promotes migration and invasion in anoikis-resistant prostate cancer cells by enhancing metabolic reprogramming. FASEB Journal, 32, 3924–3935.
Park, S. Y., Lee, Y.-K., Kim, H. J., Park, O. J., & Kim, Y. M. (2016). AMPK interacts with β-catenin in the regulation of hepatocellular carcinoma cell proliferation and survival with selenium treatment. Oncology Reports, 35, 1566–1572.
Lu, D., & Carson, D. A. (2010). Repression of beta-catenin signaling by PPAR gamma ligands. European Journal of Pharmacology, 636, 198–202.
Liu, J., Wand, H., Zuo, Y., & Farmer, S. R. (2006). Functional interaction between peroxisome proliferator-activated receptor gamma and beta-catenin. Molecular and Cellular Biology, 26, 5827–5837.
Li, Y., Zhao, T., Li, H., & Li, T. (2015). Analysis on traditional Chinese medicine’s medication rule in prescriptions for the scleroderma based on data mining. Medical Research and Education, 33, 29–34+41.
Li, J., Ding, M., & Mou, P., et al. (2014). Effects of asiaticoside on proliferation, collagen synthesis and TGF-β1 secretion of fibroblasts inpatients with systemic sclerosis. Jiangsu Medical Journal, 40, 2387–2389.
Zhang, Y., Gu, F., & Wang, Y. (2017). Clinical study of total glucosides of paeony for adjuvant therapy of systemic sclerosis. Chinese Journal of Integrated Traditional and Western Medicine, 37, 785–788.
Sozio, M. S., Lu, C., & Zeng, Y. (2011). Activated AMPK inhibits PPAR-{alpha} and PPAR-{gamma} transcriptional activity in hepatoma cells. American Journal of Physiology - Gastrointestinal and Liver Physiology, 301, G739–G747.
King, T. D., Song, L., & Jope, R. S. (2006). AMP-activated protein kinase (AMPK) activating agents cause dephosphorylation of Akt and glycogen synthase kinase-3. Biochemical Pharmacology, 71, 1637–1647.
Wu, J., Du, J., & Fu, X. (2016). Iciartin, a novel FASN inhibitor, exerts anti-melanoma activities through IGF-1R/STAT3 signaling. Oncotarget, 7, 51251–51269.
Denton, C. P., & Khanna, D. (2017). Systemic sclerosis. The Lancet, 390, 1685–1699.
Liang, R., Kagwiria, R., & Zehender, A., et al. (2019). Acyltransferase skinny hedgehog regulates TGFbeta-dependent fibroblast activation in SSc. Annals of the Rheumatic Diseases, 78, 1269–1273.
Dees, C., Tomcik, M., & Zerr, P., et al. (2011). Notch signalling regulates fibroblast activation and collagen release in systemic sclerosis. Annals of the Rheumatic Diseases, 70, 1304–1310.
Xu, Y., Li, L., & Tang, J., et al. (2019). Icariin promotes osteogenic differentiation by suppressing Notch signaling. European Journal of Pharmacology, 865, 172794.
Park, M. J., Moon, S.-J., & Lee, E.-J., et al. (2018). IL-1-IL-17 signaling axis contributes to fibrosis and inflammation in two different murine models of systemic sclerosis. Frontiers in Immunology, 9, 1611.
Balanescu, P., Balanescu, E., & Balanescu, A. (2017). IL-17 and Th17 cells in systemic sclerosis: a comprehensive review. Romanian Journal of Internal Medicine, 55, 198–204.
Liao, J., Liu, Y., & Wu, H., et al. (2016). The role of icaritin in regulating Foxp3/IL17a balance in systemic lupus erythematosus and its effects on the treatment of MRL/lpr mice. Clinical Immunology, 162, 74–83.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (Grant no. 81673917), and Fujian provincial health technology project (No. 2016 -ZQN-14).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Li, M., Liu, Q., He, S. et al. Icaritin Inhibits Skin Fibrosis through Regulating AMPK and Wnt/β-catenin Signaling. Cell Biochem Biophys 79, 231–238 (2021). https://doi.org/10.1007/s12013-020-00952-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-020-00952-z