Abstract
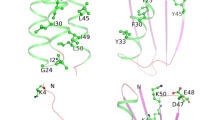
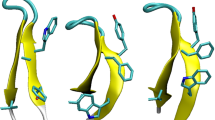
The replica-exchange Monte Carlo method based on the single amino acid potential (SAAP) force field, i.e., REMC/SAAP3D, was recently developed by our group for the molecular simulation of short peptides. In this study, the method has been improved by applying a distance-dependent dielectric (DDD) constant and extended to the peptides containing d-amino acid (AA) residues. For chignolin (10 AAs), a sigmoidal DDD model reasonably allocated the native-like β-hairpin structure with all-atom root mean square deviation (RMSD) = 2.0 Å as a global energy minimum. The optimal DDD condition was subsequently applied for Trp-cage (20 AAs) and its G10q mutant. The native-like α-rich folded structures with main-chain RMSD = 3.7 and 3.8 Å were obtained as global energy minima for Trp-cage and G10q, respectively. The results suggested that the REMC/SAAP3D method with the sigmoidal DDD model is useful for structural prediction for the short peptides comprised of up to 20 AAs. In addition, the relative contributions of SAAP to the total energy (%SAAP) were evaluated by energetic component analysis. The ratios of %SAAP were about 40 and 20% for chignolin and Trp-cage (or G10q), respectively. It was proposed that SAAP is more important for the secondary structure formation than for the assembly to a higher-order folded structure, in which the attractive van der Waals interaction may play a more important role.






Similar content being viewed by others
Data Availability
Supplementary data are available from the journal web page.
Code Availability
The program code of REMC/SAAP3D is available from the corresponding author at a request.
References
Scheraga HA, Khalili M, Liwo A (2007) Protein-folding dynamics: overview of molecular simulation techniques. Annu Rev Phys Chem 58:57–83. https://doi.org/10.1146/annurev.physchem.58.032806.104614
Adessi C, Soto C (2005) Converting a peptide into a drug: strategies to improve stability and bioavailability. Curr Med Chem 9:963–978. https://doi.org/10.2174/0929867024606731
Molinski TF, Dalisay DS, Lievens SL, Saludes JP (2009) Drug development from marine natural products. Nat Rev Drug Discov 8:69–85. https://doi.org/10.1038/nrd2487
Vlieghe P, Lisowski V, Martinez J, Khrestchatisky M (2010) Synthetic therapeutic peptides: science and market. Drug Discov Today 15:40–56. https://doi.org/10.1016/j.drudis.2009.10.009
Maupetit J, Derreumaux P, Tufféry P (2010) A fast method for large-scale de novo peptide and miniprotein structure prediction. J Comput Chem 31:726–738. https://doi.org/10.1002/jcc.21365
Nguyen PH, Li MS, Derreumaux P (2011) Effects of all-atom force fields on amyloid oligomerization: replica exchange molecular dynamics simulations of the Aβ16-22 dimer and trimer. Phys Chem Chem Phys 13:9778–9788. https://doi.org/10.1039/c1cp20323a
Trellet M, Melquiond ASJ, Bonvin AMJJ (2013) A unified conformational selection and induced fit approach to protein-peptide docking. PLoS ONE 8:e58769. https://doi.org/10.1371/journal.pone.0058769
Lensink MF, Velankar S, Wodak SJ (2017) Modeling protein–protein and protein–peptide complexes: CAPRI 6th edition. Proteins Struct Funct Bioinf 85:359–377. https://doi.org/10.1002/prot.25215
Ulmschneider JP, Ulmschneider MB (2018) Molecular dynamics simulations are redefining our view of peptides interacting with biological membranes. Acc Chem Res 51:1106–1116. https://doi.org/10.1021/acs.accounts.7b00613
Weiner SJ, Kollman PA, Singh UC et al (1984) A new force field for molecular mechanical simulation of nucleic acids and proteins. J Am Chem Soc 106:765–784. https://doi.org/10.1021/ja00315a051
Weiner SJ, Kollman PA, Nguyen DT, Case DA (1986) An all atom force field for simulations of proteins and nucleic acids. J Comput Chem 7:230–252. https://doi.org/10.1002/jcc.540070216
Brooks BR, Bruccoleri RE, Olafson BD et al (1983) CHARMM: a program for macromolecular energy, minimization, and dynamics calculations. J Comput Chem 4:187–217. https://doi.org/10.1002/jcc.540040211
Scott WRP, Hünenberger PH, Tironi IG et al (1999) The GROMOS biomolecular simulation program package. J Phys Chem A 103:3596–3607. https://doi.org/10.1021/jp984217f
Jorgensen WL, Maxwell DS, Tirado-Rives J (1996) Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J Am Chem Soc 118:11225–11236. https://doi.org/10.1021/ja9621760
Halgren TA (1996) Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J Comput Chem 17:490–519. https://doi.org/10.1002/(SICI)1096-987X(199604)17:5/6%3c490::AID-JCC1%3e3.0.CO;2-P
Sun H (1998) Compass: an ab initio force-field optimized for condensed-phase applications—overview with details on alkane and benzene compounds. J Phys Chem B 102:7338–7364. https://doi.org/10.1021/jp980939v
Möllhoff M, Sternberg U (2001) Molecular mechanics with fluctuating atomic charges—a new force field with a semi-empirical charge calculation. J Mol Model 7:90–102. https://doi.org/10.1007/S008940100008
Momany FA, McGuire RF, Burgess AW, Scheraga HA (1975) Energy parameters in polypepltides. VII. Geometric parameters, partial atomic charges, nonbonded interactions, hydrogen bond interactions, and intrinsic torsional potentials for the naturally occurring amino acids. J Phys Chem 79:2361–2381. https://doi.org/10.1021/j100589a006
Némethy G, Gibson KD, Palmer KA et al (1992) Energy parameters in polypeptides. 10. Improved geometrical parameters and nonbonded interactions for use in the ECEPP/3 algorithm, with application to proline-containing peptides. J Phys Chem 96:6472–6484. https://doi.org/10.1021/j100194a068
Robertson MJ, Tirado-Rives J, Jorgensen WL (2015) Improved peptide and protein torsional energetics with the OPLS-AA force field. J Chem Theory Comput 11:3499–3509. https://doi.org/10.1021/acs.jctc.5b00356
Vanommeslaeghe K, MacKerell AD (2015) CHARMM additive and polarizable force fields for biophysics and computer-aided drug design. Biochim Biophys Acta Gen Subj 1850:861–871. https://doi.org/10.1016/j.bbagen.2014.08.004
Maier JA, Martinez C, Kasavajhala K et al (2015) ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB. J Chem Theory Comput 11:3696–3713. https://doi.org/10.1021/acs.jctc.5b00255
Harder E, Damm W, Maple J et al (2016) OPLS3: a force field providing broad coverage of drug-like small molecules and proteins. J Chem Theory Comput 12:281–296. https://doi.org/10.1021/acs.jctc.5b00864
Huang J, Rauscher S, Nawrocki G et al (2016) CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat Methods 14:71–73. https://doi.org/10.1038/nmeth.4067
Bixon M, Lifson S (1967) Potential functions and conformations in cycloalkanes. Tetrahedron 23:769–784. https://doi.org/10.1016/0040-4020(67)85023-3
Levitt M (2001) The birth of computational structural biology. Nat Struct Biol 8:392–393. https://doi.org/10.1038/87545
Iwaoka M, Tomoda S (2003) The SAAP force field. A simple approach to a new all-atom protein force field by using single amino acid potential (SAAP) functions in various solvents. J Comput Chem 24:1192–1200. https://doi.org/10.1002/jcc.10259
Iwaoka M, Kimura N, Yosida D, Minezaki T (2009) The SAAP force field: development of the single amino acid potentials for 20 proteinogenic amino acids and Monte Carlo molecular simulation for short peptides. J Comput Chem 30:2039–2055. https://doi.org/10.1002/jcc.21196
Dedachi K, Shimosato T, Minezaki T, Iwaoka M (2013) Toward structure prediction for short peptides using the improved SAAP force field parameters. J Chem. https://doi.org/10.1155/2013/407862
Iwaoka M, Suzuki T, Shoji Y et al (2017) Development of SAAP3D force field and the application to replica-exchange Monte Carlo simulation for chignolin and C-peptide. J Comput Aided Mol Des 31:1039–1052. https://doi.org/10.1007/s10822-017-0084-8
Honda S, Yamasaki K, Sawada Y, Morii H (2004) 10 Residue folded peptide designed by segment statistics. Structure 12:1507–1518. https://doi.org/10.1016/j.str.2004.05.022
Osterhout JJ, Baldwin RL, York EJ et al (1989) 1H NMR studies of the solution conformations of an analogue of the C-peptide of ribonuclease A. Biochemistry 28:7059–7064. https://doi.org/10.1021/bi00443a042
Guenot J, Kollman PA (1992) Molecular dynamics studies of a DNA-binding protein: 2. An evaluation of implicit and explicit solvent models for the molecular dynamics simulation of the Escherichia coli trp repressor. Protein Sci 1:1185–1205. https://doi.org/10.1002/pro.5560010912
Hingerty BE, Ritchie RH, Ferrell TL, Turner JE (1985) Dielectric effects in biopolymers: the theory of ionic saturation revisited. Biopolymers 24:427–439. https://doi.org/10.1002/bip.360240302
Ramstein J, Lavery R (1988) Energetic coupling between DNA bending and base pair opening. Proc Natl Acad Sci U S A 85:7231–7235. https://doi.org/10.1073/pnas.85.19.7231
Young MA, Jayaram B, Beveridge DL (1998) Local dielectric environment of B-DNA in solution: results from a 14 ns molecular dynamics trajectory. J Phys Chem B 102:7666–7669. https://doi.org/10.1021/jp9823188
Neidigh JW, Fesinmeyer RM, Andersen NH (2002) Designing a 20-residue protein. Nat Struct Biol 9:425–430. https://doi.org/10.1038/nsb798
Rodriguez-Granillo A, Annavarapu S, Zhang L et al (2011) Computational design of thermostabilizing D-amino acid substitutions. J Am Chem Soc 133:18750–18759. https://doi.org/10.1021/ja205609c
Terada T, Satoh D, Mikawa T et al (2008) Understanding the roles of amino acid residues in tertiary structure formation of chignolin by using molecular dynamics simulation. Proteins 73:621–631. https://doi.org/10.1002/prot.22100
Harada R, Kitao A (2011) Exploring the folding free energy landscape of a β-hairpin miniprotein, chignolin, using multiscale free energy landscape calculation method. J Phys Chem B 115:8806–8812. https://doi.org/10.1021/jp2008623
Harada R, Takano Y, Shigeta Y (2015) Efficient conformational sampling of proteins based on a multi-dimensional TaBoo SeArch algorithm: an application to folding of chignolin in explicit solvent. Chem Phys Lett 630:68–75. https://doi.org/10.1016/j.cplett.2015.04.039
Huang YMM, McCammon JA, Miao Y (2018) Replica exchange Gaussian accelerated molecular dynamics: improved enhanced sampling and free energy calculation. J Chem Theory Comput 14:1853–1864. https://doi.org/10.1021/acs.jctc.7b01226
Aida H, Shigeta Y, Harada R (2020) Regenerations of initial velocities in parallel cascade selection molecular dynamics (PaCS-MD) enhance the conformational transitions of proteins. Chem Lett 49:798–801. https://doi.org/10.1246/cl.200196
Maruyama Y, Koroku S, Imai M et al (2020) Mutation-induced change in chignolin stability from π-turn to α-turn. RSC Adv 10:22797–22808. https://doi.org/10.1039/d0ra01148g
Pitera JW, Swope W (2003) Understanding folding and design: replica-exchange simulations of “Trp-cage” miniproteins. Proc Natl Acad Sci U S A 100:7587–7592. https://doi.org/10.1073/pnas.1330954100
Mou L, Jia X, Gao Y et al (2014) Folding simulation of Trp-cage utilizing a new AMBER compatible force field with coupled main chain torsions. J Theor Comput Chem 13:1450026. https://doi.org/10.1142/S0219633614500266
Chalyavi F, Schmitz AJ, Tucker MJ (2020) Unperturbed detection of the dynamic structure in the hydrophobic core of Trp-Cage via two-dimensional infrared spectroscopy. J Phys Chem Lett 11:832–837. https://doi.org/10.1021/acs.jpclett.9b03706
Kato K, Nakayoshi T, Fukuyoshi S et al (2017) Validation of molecular dynamics simulations for prediction of three-dimensional structures of small proteins. Molecules 22:1716. https://doi.org/10.3390/molecules22101716
Shao J, Tanner SW, Thompson N, Cheatham TE (2007) Clustering molecular dynamics trajectories: 1. Characterizing the performance of different clustering algorithms. J Chem Theory Comput 3:2312–2334. https://doi.org/10.1021/ct700119m
Ooi T, Oobatake M, Némethy G, Scheraga HA (1987) Accessible surface areas as a measure of the thermodynamic parameters of hydration of peptides. Proc Natl Acad Sci U S A 84:3086–3090. https://doi.org/10.1073/pnas.84.10.3086
Zhou H-X, Pang X (2018) Electrostatic interactions in protein structure, folding, binding, and condensation. Chem Rev 118:1691–1741. https://doi.org/10.1021/acs.chemrev.7b00305
Iwaoka M, Okada M, Tomoda S (2002) Solvent effects on the φ-ψ potential surfaces of glycine and alanine dipeptides studied by PCM and I-PCM methods. J Mol Struct Theochem 586:111–124. https://doi.org/10.1016/S0166-1280(02)00076-3
Iwaoka M, Yosida D, Kimura N (2006) Importance of the single amino acid potential in water for secondary and tertiary structures of proteins. J Phys Chem B 110:14475–14482. https://doi.org/10.1021/jp062196g
Smith LJ, Fiebig KM, Schwalbe H, Dobson CM (1996) The concept of a random coil. Residual structure in peptides and denatured proteins. Fold Des 1:R95–R106. https://doi.org/10.1016/S1359-0278(96)00046-6
Fiebig KM, Schwalbe H, Buck M et al (1996) Toward a description of the conformations of denatured states of proteins. Comparison of a random coil model with NMR measurements. J Phys Chem 100:2661–2666. https://doi.org/10.1021/jp952747v
Head-Gordon T, Head-Gordon M, Frisch MJ et al (1991) Theoretical study of blocked glycine and alanine peptide analogues. J Am Chem Soc 113:5989–5997. https://doi.org/10.1021/ja00016a010
Shang HS, Head-Gordon T (1994) Stabilization of helices in glycine and alanine dipeptides in a reaction field model of solvent. J Am Chem Soc 116:1528–1532. https://doi.org/10.1021/ja00083a042
Gould IR, Cornell WD, Hillier IH (1994) A quantum mechanical investigation of the conformational energetics of the alanine and glycine dipeptides in the gas phase and in aqueous solution. J Am Chem Soc 116:9250–9256. https://doi.org/10.1021/ja00099a048
Hudáky I, Hudáky P, Perczel A (2004) Solvation model induced structural changes in peptides. A quantum chemical study on Ramachandran surfaces and conformers of alanine diamide using the polarizable continuum model. J Comput Chem 25:1522–1531. https://doi.org/10.1002/jcc.20073
Bartlett GJ, Choudhary A, Raines RT, Woolfson DN (2010) n→π* interactions in proteins. Nat Chem Biol 6:615–620. https://doi.org/10.1038/nchembio.406
Newberry RW, Raines RT (2016) A prevalent intraresidue hydrogen bond stabilizes proteins. Nat Chem Biol 12:1084–1088. https://doi.org/10.1038/nchembio.2206
León I, Alonso ER, Cabezas C et al (2019) Unveiling the n→π* interactions in dipeptides. Commun Chem 2:3. https://doi.org/10.1038/s42004-018-0103-2
Muchowska KB, Pascoe DJ, Borsley S et al (2020) Reconciling electrostatic and n→π* orbital contributions in carbonyl interactions. Angew Chem Int Ed 59:14602–14608. https://doi.org/10.1002/anie.202005739
Shimodaira S, Takei T, Hojo H, Iwaoka M (2018) Synthesis of selenocysteine-containing cyclic peptides via tandem N-to-S acyl migration and intramolecular selenocysteine-mediated native chemical ligation. Chem Commun 54:11737–11740. https://doi.org/10.1039/c8cc06805d
Funding
This work was supported by Grant-in-Aid for Scientific Research on Innovative Areas (No. 2120005) from the Ministry of Education, Culture, Sports, Science and Technology, Japan (MI).
Author information
Authors and Affiliations
Contributions
MI organized the SAAP project and prepared the manuscript. KY modified the source code and carried out simulations and data analysis. TS managed the SAAP force field parameters, including those for D-amino acids.
Corresponding author
Ethics declarations
Conflicts of interest
Not applicable.
Additional information
This paper is dedicated to late Dr. Harold A. Scheraga.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Iwaoka, M., Yoshida, K. & Shimosato, T. Application of a Distance-Dependent Sigmoidal Dielectric Constant to the REMC/SAAP3D Simulations of Chignolin, Trp-Cage, and the G10q Mutant. Protein J 39, 402–410 (2020). https://doi.org/10.1007/s10930-020-09936-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-020-09936-7