Abstract
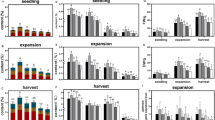
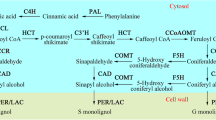
Shading can effectively reduce photoinhibition and improve the quality of tea. Lignin is one of the most important secondary metabolites that play vital functions in plant growth and development. However, little is known about the relationship between shading and xylogenesis in tea plant. To investigate the effects of shading on lignin accumulation in tea plants, ‘Longjing 43’ was treated with no shading (S0), 40% (S1) and 80% (S2) shading treatments, respectively. The leaf area and lignin content of tea plant leaves decreased under shading treatments (especially S2). The anatomical characteristics showed that lignin is mainly distributed in the xylem of tea leaves. Promoter analysis indicated that the genes involved in lignin pathway contain several light recognition elements. The transcript abundances of 12 lignin-associated genes were altered under shading treatments. Correlation analysis indicated that most genes showed strong positive correlation with lignin content, and CsPAL, Cs4CL, CsF5H, and CsLAC exhibited significant positively correlation under 40% and 80% shading treatments. The results showed that shading may have an important effect on lignin accumulation in tea leaves. This work will potentially helpful to understand the regulation mechanism of lignin pathway under shading treatment, and provide reference for reducing lignin content and improving tea quality through shading treatment in field operation.









Similar content being viewed by others
References
Albert NW, Lewis DH, Zhang H, Irving LJ, Jameson PE, Davies KM (2009) Light-induced vegetative anthocyanin pigmentation in Petunia. J Exp Bot 60:2191–2202
Ali MB, McNear DH Jr (2014) Induced transcriptional profiling of phenylpropanoid pathway genes increased flavonoid and lignin content in Arabidopsis leaves in response to microbial products. BMC Plant Biol 14:84
Boerjan W, Ralph J, Baucher M (2003) Lignin biosynthesis. Annu Rev Plant Biol 54:519–546
Boudet AM (2000) Lignins and lignification: selected issues. Plant Physiol Biochem 38:81–96
Castillon A, Hui S, Huq E (2007) Phytochrome Interacting Factors: central players in phytochrome-mediated light signaling networks. Trends Plant Sci 12:514–521
Cervilla LM, Rosales MA, Rubio-Wilhelmi MM, Sánchez-Rodríguez E, Blasco B, Ríos JJ, Romero L, Ruiz JM (2009) Involvement of lignification and membrane permeability in the tomato root response to boron toxicity. Plant Sci 176:545–552
Cheng X, Li M, Li D, Zhang J, Jin Q, Sheng L, Cai Y, Lin Y (2017) Characterization and analysis of CCR and CAD gene families at the whole-genome level for lignin synthesis of stone cells in pear (Pyrus bretschneideri) fruit. Biol Open 6:1602–1613
Chory J (1997) Light modulation of vegetative development. Plant Cell 9:1225–1234
de Jong F, Hanley SJ, Beale MH, Karp A (2015) Characterisation of the willow phenylalanine ammonia-lyase (PAL) gene family reveals expression differences compared with poplar. Phytochemistry 117:90–97
Dolatkhahi A, Mansour M (2013) Shading impact on qualitative characteristics and chlorophyll content of cut rose (Rosa hybrida cv Avalanche). Journal of Ornamental Plants 3:215–220
Donaldson LA, Knox JP (2012) Localization of Cell Wall Polysaccharides in Normal and Compression Wood of Radiata Pine: relationships with Lignification and Microfibril Orientation. Plant Physiol 158:642–653
Eungwanichayapant PD, Popluechai S (2009) Accumulation of catechins in tea in relation to accumulation of mRNA from genes involved in catechin biosynthesis. Plant Physiol Biochem 47:94–97
Fan Y, Chen J, Cheng Y, Raza MA, Wu X, Wang Z, Liu Q, Wang R, Wang X, Yong T (2018) Effect of shading and light recovery on the growth, leaf structure, and photosynthetic performance of soybean in a maize-soybean relay-strip intercropping system. PLoS ONE 13:e0198159
Goujon T, Sibout R, Eudes A, MacKay J, Joulanin L (2003) Genes involved in the biosynthesis of lignin precursors in Arabidopsis thaliana. Plant Physiol Biochem 41:677–687
Hu WJ, Harding SA, Lung J, Popko JL, Ralph J, Stokke DD, Tsai CJ, Chiang VL (1999) Repression of lignin biosynthesis promotes cellulose accumulation and growth in transgenic trees. Nat Biotechnol 17:808–812
Hui S, Fu C, Xiao X, Ray T, Tang Y, Wang Z, Fang C (2009) Developmental control of lignification in stems of lowland switchgrass variety alamo and the effects on saccharification efficiency. Bioenerg Res 2:233–245
Ithal N, Recknor J, Nettleton D, Maier T, Baum TJ, Mitchum MG (2007) Developmental transcript profiling of cyst nematode feeding cells in soybean roots. Mol Plant Microbe Int 20:510–525
Koretskaya TF, Zaprometov MN (1975) Phenolic compounds in cultures of tissues of tea plants and the effect of light on their synthesis. Soviet Plant Physiol. https://doi.org/10.1021/bp025539a
Li J, Ou-Lee TM, Raba R, Amundson RG, Last RL (1993) Arabidopsis flavonoid mutants are hypersensitive to UV-B irradiation. Plant Cell 5:171–179
Li H, Liu ZW, Wu ZJ, Wang YX, Teng RM, Zhuang J (2018) Differentially expressed protein and gene analysis revealed the effects of temperature on changes in ascorbic acid metabolism in harvested tea leaves. Hortic Res 5:65
Liang YR, Liu ZS, Xu YR, Hu YL (2010) A study on chemical composition of two special green teas (Camellia sinensis). J Sci Food Agric 53:541–548
Liu JX, Feng K, Wang GL, Xu ZS, Wang F, Xiong AS (2018a) Elevated CO2 induces alteration in lignin accumulation in celery (Apium graveolens L.). Plant Physiol Biochem 127:310–319
Liu Q, Luo L, Zheng L (2018b) Lignins: biosynthesis and biological Functions in Plants. Int J Mol Sci 19:335
Luo YP (2008) Tea cultivation. China Agriculture Press, Beijing
Magali L, Patrice D, Gert T, Kathleen M, Yves M, Yves VDP, Pierre R, Stephane R (2002) PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res 30:325–327
Matus JT, Loyola R, Vega A, Peña-Neira A, Bordeu E, Arce-Johnson P, Alcalde JA (2009) Post-veraison sunlight exposure induces myb-mediated transcriptional regulation of anthocyanin and flavonol synthesis in berry skins of Vitis vinifera. J Exp Bot 60:853–867
Mellway RD, Tran LT, Prouse MB, Campbell MM, Constabel CP (2009) The wound-, pathogen-, and ultraviolet B-responsive MYB134 gene encodes an R2R3 MYB transcription factor that regulates proanthocyanidin synthesis in poplar. Plant Signal Behav 150:924–941
Mo KK, Jung Nam C, Jiyoung K, Jeong Kee K, Gook YL, Jun LS, Young-Shick H, Choong Hwan L (2010) Metabolomics analysis reveals the compositional differences of shade grown tea (Camellia sinensis L.). J Agric Food Chem 58:418–426
Monteiro EB, Silva CCD, Silva ACD, Souza APD (2014) Estimating emission of leaves seedlings forest in different shading levels, at conditions of transition Amazon-Cerrado, Brazil. Am J Plant Sci 5:2330–2341
Monteith JL (1965) Light distribution and photosynthesis in field crops. Ann Bot 29:17–37
Muir JP, Bow JR, Boggs LL (2009) Response of two perennial herbaceous Texas legumes to shade. Nat Plants J 10:252–261
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Qiao Z, Dixon RA (2011) Transcriptional networks for lignin biosynthesis: more complex than we thought? Trends Plant Sci 16:227–233
Qin ZM, Tanui J, Feng WY, Wang YH, Xiao RL, Xing-Hui LI (2011) Effects of shading on yield index and biochemical components of tea in hilly tea plantation. J Nanjing Agric Univ 34:47–52
Que F, Wang GL, Feng K, Xu ZS, Wang F, Xiong AS (2018) Hypoxia enhances lignification and affects the anatomical structure in hydroponic cultivation of carrot taproot. Plant Cell Rep 37:1021–1032
Riboulet C, Guillaumie S, Méchin V, Bosio M, Pichon M, Goffner D, Lapierre C, Pollet B, Lefevre B, Martinant JP (2009) Kinetics of phenylpropanoid gene expression in maize growing internodes: relationships with cell wall deposition. Crop Sci 49:211–223
Rogers LA, Campbell MM (2004) The genetic control of lignin deposition during plant growth and development [Review]. New Phytol 164:17–30
Ruben V, Brecht D, Kris M, John R, Wout B (2010) Lignin biosynthesis and structure. Plant Physiol 153:895–905
Ruben V, Kris M, Chiarina D, Paula O, Grabber JH, John R, Wout B (2012) Metabolic engineering of novel lignin in biomass crops. New Phytol 196:978–1000
Saijo R (1980) Effect of shade treatment on biosynthesis of catechins in tea plants. Plant Cell Physiol 21:989–998
Saito K, Yonekura-Sakakibara K, Nakabayashi R, Higashi Y, Yamazaki M, Tohge T, Fernie AR (2013) The flavonoid biosynthetic pathway in Arabidopsis: structural and genetic diversity. Plant Physiol Biochem 72:21–34
Shadle G, Chen F, Reddy MSS, Jackson L, Jin N, Dixon RA (2007) Down-regulation of hydroxycinnamoyl CoA: shikimate hydroxycinnamoyl transferase in transgenic alfalfa affects lignification, development and forage quality. Phytochemistry 68:1521–1529
Tian YY, Zhang LX, Zhang ZQ, Qiao MM, Fan YG (2017) Effects of shading in summer and autumn on physiological and biochemical characteristics of ‘Huangjinya’ in Shandong Province, China. Chin J Appl Ecol 28:789–796
Wang YS, Gao LP, Shan Y, Liu YJ, Tian YW, Xia T (2012a) Influence of shade on flavonoid biosynthesis in tea (Camellia sinensis (L.) O. Kuntze). Sci Hortic 141:7–16
Wang YS, Gao LP, Wang ZR, Liu YJ, Sun ML, Yang DQ, Wei CL, Shan Y, Xia T (2012b) Light-induced expression of genes involved in phenylpropanoid biosynthetic pathways in callus of tea (Camellia sinensis (L.) O. Kuntze). Sci Hortic 133:72–83
Wang GL, Huang Y, Zhang XY, Xu ZS, Wang F, Xiong AS (2016) Transcriptome-based identification of genes revealed differential expression profiles and lignin accumulation during root development in cultivated and wild carrots. Plant Cell Rep 35:1743–1755
Wang GL, Que F, Xu ZS, Wang F, Xiong AS (2017) Exogenous gibberellin enhances secondary xylem development and lignification in carrot taproot. Protoplasma 254:839–848
Wang YX, Teng RM, Wang WL, Wang Y, Shen W, Zhuang J (2018) Identification of genes revealed differential expression profiles and lignin accumulation during leaf and stem development in tea plant (Camellia sinensis (L.) O. Kuntze). Protoplasma 256:359–370
Wei C, Yang H, Wang S, Zhao J, Liu C, Gao L, Xia E, Lu Y, Tai Y, She G, Sun J, Cao H, Tong W, Gao Q, Li Y, Deng W, Jiang X, Wang W, Chen Q, Zhang S, Li H, Wu J, Wang P, Li P, Shi C, Zheng F, Jian J, Huang B, Shan D, Shi M, Fang C, Yue Y, Li F, Li D, Wei S, Han B, Jiang C, Yin Y, Xia T, Zhang Z, Bennetzen JL, Zhao S, Wan X (2018) Draft genome sequence of Camellia sinensis var. sinensis provides insights into the evolution of the tea genome and tea quality. P Natl Acad Sci USA 11:E4151–E4158
Wu ZJ, Li XH, Liu ZW, Li H, Wang YX, Zhuang J (2016) Transcriptome-wide identification of Camellia sinensis WRKY transcription factors in response to temperature stress. Mol Genet Genomics 291:255–269
Xu B, Escamilla-Treviño LL, Sathitsuksanoh N, Shen Z, Shen H, Percival Zhang YH, Dixon RA, Zhao B (2011) Silencing of 4-coumarate:coenzyme A ligase in switchgrass leads to reduced lignin content and improved fermentable sugar yields for biofuel production. New Phytol 192:611–625
Xu ZS, Huang Y, Wang F, Song X, Wang GL, Xiong AS (2014) Transcript profiling of structural genes involved in cyanidin-based anthocyanin biosynthesis between purple and non-purple carrot (Daucus carota L.) cultivars reveals distinct patterns. BMC Plant Biol 14:262
Yu BF, Liu Y, Pan YJ, Liu J, Wang HZ, Tang ZH (2018) Light enhanced the biosynthesis of terpenoid indole alkaloids to meet the opening of cotyledons in process of photomorphogenesis of Catharanthus roseus. Plant Growth Regul 84:617–626
Zagoskina NV, Dubravina GA, Alyavina AK, Goncharuk EA (2003) Effect of ultraviolet (UV-B) radiation on the formation and localization of phenolic compounds in tea plant callus cultures. Russ J Plant Phys 50:270–275
Zhang LX, Guo QS, Chang QS, Zhu ZB, Liu L, Chen YH (2015) Chloroplast ultrastructure, photosynthesis and accumulation of secondary metabolites in Glechoma longituba in response to irradiance. Photosynthetica 53:144–153
Zheng M, Chen J, Shi Y, Li Y, Yin Y, Yang D, Luo Y, Pang D, Xu X, Li W, Ni J, Wang Y, Wang Z, Li Y (2017) Manipulation of lignin metabolism by plant densities and its relationship with lodging resistance in wheat. Sci Rep 7:41805
Acknowledgements
The research was supported by the National Natural Science Foundation of China (31870681), Priority Academic Program Development of Jiangsu Higher Education Institutions Project (PAPD).
Author information
Authors and Affiliations
Contributions
ZJ, WYX and TRM designed this research. TRM, WYX, LH, LSJ, and LH performed the experiments. WYX conducted the data analysis. TRM and WYX wrote the manuscript. ZJ and TRM revised the paper. All the authors contributed to improving the paper and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Communicated by Stefan Hohmann.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Teng, RM., Wang, YX., Li, H. et al. Effects of shading on lignin biosynthesis in the leaf of tea plant (Camellia sinensis (L.) O. Kuntze). Mol Genet Genomics 296, 165–177 (2021). https://doi.org/10.1007/s00438-020-01737-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-020-01737-y