Abstract
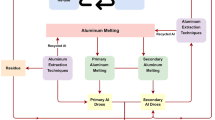
High-energy consumption in primary aluminum production (from bauxite ores) makes secondary aluminum production (from metallic raw materials such as scrap and dross) very critical. Aluminum dross, as a waste, arises in pyrometallurgical processes, and it is the mixture of metallic Al, Al2O3, other metal oxides, metal halides, and some other compounds like AlN. Aluminum dross has two subgroups (white and black) with respect to its metallic aluminum content. White dross is a secondary aluminum resource because of its high metallic aluminum content, whereas black dross, having low metallic aluminum content, is generally disposed in dumping areas. In the present study, it was aimed to develop a simple technique to valorize aluminum black dross in the form of alumina-based ceramic materials through pyrometallurgical (calcination) and hydrometallurgical (leaching) operations. Moreover, thermochemical simulations were conducted by using HSC Chemistry 6.12 software to simulate calcination conditions. After leaching experiments, an alumina-based ceramic containing approximately 36.8% Al2O3, 61.4% MgAl2O4 with 1.8% Na2FeO4, which might be available to use as a spinel refractory in steel smelting furnaces, was obtained in the experiment carried out in a solution containing 10 mL H2SO4 and 40 mL distilled water (3.5 M). Moreover, the sinterability of the alumina-based ceramic was investigated at the temperatures from 1350 to 1550 °C. In the sample which was sintered at 1550 °C, a density value of 3.15 g cm−3 and a hardness value of 69.58 HV were measured.















Similar content being viewed by others
Data Availability
Available.
References
Velasco E, Nino J (2011) Recycling of aluminium scrap for secondary Al-Si alloys. Waste Manag Res 29:686–693. https://doi.org/10.1177/0734242X10381413
Davis JR (1993) ASM specialty handbook: aluminum and aluminum alloys. ASM Int:3–12. https://doi.org/10.1017/CBO9781107415324.004
Tan RBH, Khoo HH (2005) An LCA study of a primary aluminum supply chain. J Clean Prod 13:607–618. https://doi.org/10.1016/j.jclepro.2003.12.022
International Aluminium Institute (2020) http://www.world-aluminium.org/. Accessed 21 Jun 2020
López-Delgado A, Robla JI, Padilla I, López-Andrés S, Romero M (2020) Zero-waste process for the transformation of a hazardous aluminum waste into a raw material to obtain zeolites. J Clean Prod 255:120178. https://doi.org/10.1016/j.jclepro.2020.120178
Tabereaux AT, Peterson RD (2014) Chapter 2.5 - Aluminum Production. In: S. Seetharaman, A. McLean, R. Guthrie, S. Sridhar (eds.) Treatise on Process Metallurgy - Volume 3: Industrial Processes, Part A. Elsevier Ltd, p 839–917
Liu G, Müller DB (2012) Addressing sustainability in the aluminum industry: a critical review of life cycle assessments. J Clean Prod 35:108–117. https://doi.org/10.1016/j.jclepro.2012.05.030
Barry TS, Uysal T, Birinci M, Erdemoğlu M (2019) Thermal and mechanical activation in acid leaching processes of non-bauxite ores available for alumina production—a review. Mining, Metall Explor 36:557–569. https://doi.org/10.1007/s42461-018-0025-7
Huang XL, El Badawy A, Arambewela M, Ford R, Barlaz M, Tolaymat T (2014) Characterization of salt cake from secondary aluminum production. J Hazard Mater 273:192–199. https://doi.org/10.1016/j.jhazmat.2014.02.035
Gil A (2007) Management of salt cake generated at secondary aluminum melting plants by disposal in a controlled landfill: characteristics of the controlled landfill and a study of environmental impacts. Environ Eng Sci 24:1234–1244. https://doi.org/10.1089/ees.2006.0123
Habashi F (1986) Principles of extractive metallurgy, Volume 3, pyrometallurgy. Gordon and Breach Science Publishers S. A., New York, London, Paris, Montreux, Tokyo
Tsakiridis PE (2012) Aluminium salt slag characterization and utilization - a review. J Hazard Mater 217–218:1–10. https://doi.org/10.1016/j.jhazmat.2012.03.052
Mahinroosta M, Allahverdi A (2018) A promising green process for synthesis of high purity activated-alumina nanopowder from secondary aluminum dross. J Clean Prod 179:93–102. https://doi.org/10.1016/j.jclepro.2018.01.079
Hu K, Reed D, Robshaw TJ, Smith RM, Ogden MD (2021) Characterisation of aluminium black dross before and after stepwise salt-phase dissolution in non-aqueous solvents. J Hazard Mater 401:123351. https://doi.org/10.1016/j.jhazmat.2020.123351
Bruckard WJ, Woodcock JT (2009) Recovery of valuable materials from aluminium salt cakes. Int J Miner Process 93:1–5. https://doi.org/10.1016/j.minpro.2009.05.002
Gil A, Korili SA (2016) Management and valorization of aluminum saline slags: current status and future trends. Chem Eng J 289:74–84. https://doi.org/10.1016/j.cej.2015.12.069
López-Delgado A, Tayibi H (2012) Can hazardous waste become a raw material? The case study of an aluminium residue: a review. Waste Manag Res 30:474–484. https://doi.org/10.1177/0734242X11422931
Beheshti R, Moosberg-Bustnes J, Akhtar S, Aune RE (2014) Black dross: processing salt removal from black dross by thermal treatment. Jom 66:2243–2252. https://doi.org/10.1007/s11837-014-1178-6
Beheshti R, Moosberg-Bustnes J, Akhtar S, Aune RE (2017) Black dross processing: utilization of black dross in the production of a ladle fluxing agent. J Sustain Metall 3:265–273. https://doi.org/10.1007/s40831-016-0076-2
Li P, Guo M, Zhang M, Wang XD, Seetharaman S (2011) Spinel synthesis from aluminium dross. Trans Institutions Min Metall Sect C Miner Process Extr Metall 120:247–250. https://doi.org/10.1179/1743285511Y.0000000007
Nguyen TTN, Lee MS, Nguyen TH (2018) Ball milling treatment of black dross for selective dissolution of alumina in sodium hydroxide leaching. Processes 6. https://doi.org/10.3390/pr6040029
Nguyen TTN, Song SJ, Lee MS (2020) Development of a hydrometallurgical process for the recovery of pure alumina from black dross and synthesis of magnesium spinel. J Mater Res Technol 9:2568–2577. https://doi.org/10.1016/j.jmrt.2019.12.087
Tsakiridis PE, Oustadakis P, Agatzini-Leonardou S (2013) Aluminium recovery during black dross hydrothermal treatment. J Environ Chem Eng 1:23–32. https://doi.org/10.1016/j.jece.2013.03.004
National Library of Medicine (2020) https://pubchem.ncbi.nlm.nih.gov/compound/Aluminum-oxide. Accessed 20 Jun 2020
Plascencia G, Jaramillo D (2017) Basic thermochemistry in materials processing. Springer International Publishing AG, Cham, p 4. https://doi.org/10.1007/978-3-319-53815-0
Martin JW, Stark TD, Thalhamer T, Gerbasi-Graf GT, Gortner RE (2013) Detection of aluminum waste reactions and waste fires. J Hazardous, Toxic, Radioact Waste 17:164–174. https://doi.org/10.1061/(asce)hz.2153-5515.0000171
Iwata T, Matsumoto T, Terakawa S, Kobayashi H (2010) Fabrication of Al2O3/Al structure by nitric acid oxidation at room temperature. Cent Eur J Phys 8:1015–1020. https://doi.org/10.2478/s11534-010-0014-z
Braulio MAL, Rigaud M, Buhr A, Parr C, Pandolfelli VC (2011) Spinel-containing alumina-based refractory castables. Ceram Int 37:1705–1724. https://doi.org/10.1016/j.ceramint.2011.03.049
Acknowledgments
The authors give thanks to Mr. Emin Akidil for providing raw material.
Funding
Yalova University, Scientific Research Projects Department under grant number 2019/YL/0002.
Author information
Authors and Affiliations
Contributions
U. Cinarli: investigation, software, writing—original draft; A. Turan: conceptualization, methodology, software, validation, supervision, writing—review and editing.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Code Availability
Available.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cinarli, U., Turan, A. Investigation of Alumina-Based Ceramic Production from Aluminum Black Dross. Mining, Metallurgy & Exploration 38, 257–267 (2021). https://doi.org/10.1007/s42461-020-00344-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42461-020-00344-0