Abstract
To balance cost and performance of geopolymers, alkalinity of activating solution is critical. Alkalinity affects condensation that determines the final gel structures, but this effect is confounded by dissolution and is not understood from direct experimental evidence. In this study, we investigated effects of alkalinity on condensation for gels synthesized via a sol–gel method that eliminates dissolution process. As alkalinity increased, particle sizes of the gels increased as indicated by SEM, Si/Al ratios of the gels decreased but polymerization extent increased as supported by FTIR, 27Al and 23Na NMR, and composition analysis. The mechanism for the effects of alkalinity was proposed accordingly: (1) increasing alkalinity lowers the Si/Al ratio (i.e., more incorporation of Al) of the resulting products probably by affecting charging conditions of the Si and Al units; (2) the presence of Al(OH)4− units promotes their condensation with nearby species to increase the extent of polymerization; (3) enhanced condensation increases particle sizes of the gels even at microstructural level. This understanding on condensation independent of dissolution provides ways to control gel structures and Si/Al ratios and thus tailor properties accordingly, as well as to suggest a strategy (by altering Si/Al ratios during condensation) to develop kinetics-controlling admixtures.

Highlights
-
Condensation of geopolymer gel was studied independently of dissolution.
-
Increasing alkalinity lowers Si/Al ratio (i.e., more incorporation of Al) of gel.
-
More incorporated Al units enhance condensation with Si species.
-
Enhanced condensation increases particle sizes of the gel at microstructural level.







Similar content being viewed by others
References
Provis J, van Deventer J (2013) Alkali activated materials: state-of-the-art report, RILEM TC 224-AAM. Vol 13. Springer Science & Business Media, Netherlands.
McLellan BC, Williams RP, Lay J, van Riessen A, Corder GD (2011) Costs and carbon emissions for geopolymer pastes in comparison to ordinary portland cement. J Clean Prod 19(9–10):1080–1090
Rattanasak U, Chindaprasirt P (2009) Influence of NaOH solution on the synthesis of fly ash geopolymer. Min Eng 22(12):1073–1078
Yao X, Zhang Z, Zhu H, Chen Y (2009) Geopolymerization process of alkali–metakaolinite characterized by isothermal calorimetry. Thermochim Acta 493(1–2):49–54
Phair JW, Van Deventer JSJ (2001) Effect of silicate activator pH on the leaching and material characteristics of waste-based inorganic polymers. Min Eng 14(3):289–304
van Jaarsveld JGS, van Deventer JSJ (1999) Effect of the alkali metal activator on the properties of fly ash-based geopolymers. Ind Eng Chem Res 38(10):3932–3941
Zhang Z, Wang H, Provis JL, Bullen F, Reid A, Zhu Y (2012) Quantitative kinetic and structural analysis of geopolymers. Part 1. The activation of metakaolin with sodium hydroxide. Thermochim Acta 539:23–33
Zhang Z, Provis JL, Reid A, Wang H (2014) Fly ash-based geopolymers: the relationship between composition, pore structure and efflorescence. Cem Concr Res 64:30–41
Rees CA, Provis JL, Lukey GC, van Deventer JSJ (2007) In situ ATR-FTIR study of the early stages of fly ash geopolymer gel formation. Langmuir 23(17):9076–9082
Puligilla S, Chen X, Mondal P (2018) Understanding the role of silicate concentration on the early-age reaction kinetics of a calcium containing geopolymeric binder. Constr Build Mater 191:206–215
Rowles M, O’Connor B (2003) Chemical optimisation of the compressive strength of aluminosilicate geopolymers synthesised by sodium silicate activation of metakaolinite. J Mater Chem 13(5):1161–1165
Steveson M, Sagoe-Crentsil K (2005) Relationships between composition, structure and strength of inorganic polymers. J Mater Sci 40(16):4247–4259
Chindaprasirt P, Jaturapitakkul C, Chalee W, Rattanasak U (2009) Comparative study on the characteristics of fly ash and bottom ash geopolymers. Waste Manag 29(2):539–543
Antonić T, Čižmek A, Subotić B (1994) Dissolution of amorphous aluminosilicate zeolite precursors in alkaline solutions. Part 2.—Mechanism of the dissolution. J Chem Soc Faraday T 90(13):1973–1977
Oelkers EH (2001) General kinetic description of multioxide silicate mineral and glass dissolution. Geochim Cosmochim Ac 65(21):3703–3719
Bunker BC (1994) Molecular mechanisms for corrosion of silica and silicate glasses. J Non Cryst Solids 179:300–308
Brady PV, Walther JV (1989) Controls on silicate dissolution rates in neutral and basic pH solutions at 25 °C. Geochim Cosmochim Ac 53(11):2823–2830
Xu H, Van Deventer JSJ (2000) Ab initio calculations on the five-membered alumino-silicate framework rings model: implications for dissolution in alkaline solutions. Comput Chem 24(3):391–404
Kinrade SD, Pole DL (1992) Effect of alkali-metal cations on the chemistry of aqueous silicate solutions. Inorg Chem 31(22):4558–4563
Provis JL, Duxson P, Lukey GC, Separovic F, Kriven WM, van Deventer JSJ (2005) Modeling speciation in highly concentrated alkaline silicate solutions. Ind Eng Chem Res 44(23):8899–8908
Duxson P, Lukey GC, Separovic F, van Deventer JSJ (2005) Effect of alkali cations on aluminum incorporation in geopolymeric gels. Ind Eng Chem Res 44(4):832–839
Silva PD, Sagoe-Crenstil K, Sirivivatnanon V (2007) Kinetics of geopolymerization: role of Al2O3 and SiO2. Cem Concr Res 37(4):512–518
Gao XX, Autef A, Prud’homme E, Michaud P, Joussein E, Rossignol S (2013) Synthesis of consolidated materials from alkaline solutions and metakaolin: existence of domains in the Al–Si–K/O ternary diagram. J Sol-Gel Sci Technol 65(2):220–229. https://doi.org/10.1007/s10971-012-2927-z
Weng L, Sagoe-Crentsil K (2007) Dissolution processes, hydrolysis and condensation reactions during geopolymer synthesis: Part I—Low Si/Al ratio systems. J Mater Sci 42(9):2997–3006
Provis JL, Van Deventer JSJ (2009) Geopolymers: structure, processing, properties and industrial applications. Woodhead Cambridge, UK
Granizo N, Palomo A, Fernandez-Jiménez A (2014) Effect of temperature and alkaline concentration on metakaolin leaching kinetics. Ceram Int 40(7, Part A):8975–8985
Chen X, Sutrisno A, Struble LJ (2018) Effects of calcium on setting mechanism of metakaolin-based geopolymer. J Am Ceram Soc 101(2):957–968
North MR, Swaddle TW (2000) Kinetics of silicate exchange in alkaline aluminosilicate solutions. Inorg Chem 39(12):2661–2665
Pelster SA, Schrader W, Schüth F (2006) Monitoring temporal evolution of silicate species during hydrolysis and condensation of silicates using mass spectrometry. J Am Ceram Soc 128(13):4310–4317
Walkley B, San Nicolas R, Sani M-A, Gehman JD, van Deventer JSJ, Provis JL (2016) Synthesis of stoichiometrically controlled reactive aluminosilicate and calcium-aluminosilicate powders. Powder Technol 297:17–33
Cui XM, Liu LP, Zheng GJ, Wang RP, Lu JP (2010) Characterization of chemosynthetic Al2O3–2SiO2 geopolymers. J Non-cryst Solids 356(2):72–76
Walkley B, San Nicolas R, Sani M-A, Rees GJ, Hanna JV, van Deventer JSJ, Provis JL (2016) Phase evolution of C-(N)-A-S-H/N-A-S-H gel blends investigated via alkali-activation of synthetic calcium aluminosilicate precursors. Cem Concr Res 89:120–135
Walkley B, San Nicolas R, Sani M-A, Gehman JD, van Deventer JSJ, Provis JL (2016) Phase evolution of Na2O–Al2O3–SiO2–H2O gels in synthetic aluminosilicate binders. Dalton T 45(13):5521–5535
Zheng G, Cui X, Zhang W, Tong Z (2009) Preparation of geopolymer precursors by sol–gel method and their characterization. J Mater Sci 44(15):3991–3996
Gevaudan JP, Wallat JD, Lama B, Srubar IIIWV (2020) PVA- and PEG-assisted sol-gel synthesis of aluminosilicate precursors for N-A-S-H geopolymer cements. J Am Ceram Soc 103:859–877
Garcia-Lodeiro I, Fernández-Jiménez A, Palomo A, Macphee DE (2010) Effect of calcium additions on N–A–S–H cementitious gels. J Am Ceram Soc 93(7):1934–1940
Garcia Lodeiro I, Fernández-Jimenez A, Palomo A, Macphee DE (2010) Effect on fresh C-S-H gels of the simultaneous addition of alkali and aluminium. Cem Concr Res 40(1):27–32
García Lodeiro I, Macphee DE, Palomo A, Fernández-Jiménez A (2009) Effect of alkalis on fresh C–S–H gels. FTIR analysis. Cem Concr Res 39(3):147–153
Garcia-Lodeiro I, Palomo A, Fernández-Jiménez A, Macphee DE (2011) Compatibility studies between N-A-S-H and C-A-S-H gels. Study in the ternary diagram Na2O–CaO–Al2O3–SiO2–H2O. Cem Concr Res 41(9):923–931
Myers RJ, L’Hôpital E, Provis JL, Lothenbach B (2015) Effect of temperature and aluminium on calcium (alumino)silicate hydrate chemistry under equilibrium conditions. Cem Concr Res 68:83–93
Martín-Garrido M, Teresa Molina-Delgado M, Martínez-Ramírez S (2019) A comparison between experimental and theoretical Ca/Si ratios in C–S–H and C–S(A)–H gels. J Sol–Gel Sci Technol. https://doi.org/10.1007/s10971-019-05097-x
Buchwald A, Zellmann HD, Kaps C (2011) Condensation of aluminosilicate gels—model system for geopolymer binders. J Non Cryst Solids 357(5):1376–1382
Chen X, Sutrisno A, Zhu L, Struble LJ (2017) Setting and nanostructural evolution of metakaolin geopolymer. J Am Ceram Soc 100(5):2285–2295
García-Lodeiro I, Fernández-Jiménez A, Blanco MT, Palomo A (2008) FTIR study of the sol-gel synthesis of cementitious gels: C-S-H and N-A-S-H. J Sol-Gel Sci Technol 45(1):63–72
Tognonvi MT, Rossignol S, Bonnet J-P (2011) Physical-chemistry of sodium silicate gelation in an alkaline medium. J Sol-Gel Sci Technol 58(3):625–635
Nguyen AD, Škvára F (2016) The influence of ambient pH on fly ash-based geopolymer. Cem Concr Com 72:275–283
Duxson P, Lukey G, Separovic F, Van Deventer J (2005) Effect of alkali cations on aluminum incorporation in geopolymeric gels. Ind Eng Chem Res 44(4):832–839
Mostowicz R, Sand LB (1982) Crystallization of ZSM-5 with relatively high (Me2/n)2O/(TPA)2O reactant ratios. Zeolites 2(2):143–146
Chen X, Meawad A, Struble LJ (2014) Method to stop geopolymer reaction. J Am Ceram Soc 97(10):3270–3275
Zhu W, Chen X, Struble LJ, Yang E-H (2018) Characterization of calcium-containing phases in alkali-activated municipal solid waste incineration bottom ash binder through chemical extraction and deconvoluted Fourier transform infrared spectra. J Clean Prod 192:782–789
Fernández-Jiménez A, Palomo A (2005) Mid-infrared spectroscopic studies of alkali-activated fly ash structure. Micropor Mesopor Mat 86(1):207–214
Tchakouté HK, Rüscher CH, Kong S, Kamseu E, Leonelli C (2016) Comparison of metakaolin-based geopolymer cements from commercial sodium waterglass and sodium waterglass from rice husk ash. J Sol-Gel Sci Technol 78(3):492–506
Walkley B, San Nicolas R, Sani M-A, Bernal SA, van Deventer JSJ, Provis JL (2017) Structural evolution of synthetic alkali-activated CaO-MgO-Na2O-Al2O3-SiO2 materials is influenced by Mg content. Cem Concr Res 99:155–171
Xue X, Stebbins JF (1993) 23Na NMR chemical shifts and local Na coordination environments in silicate crystals, melts and glasses. Phys Chem Min 20(5):297–307
Merzbacher CI, Sherriff BL, Hartman JS, White WB (1990) A high-resolution 29Si and 27Al NMR study of alkaline earth aluminosilicate glasses. J Non-Cryst Solids 124(2–3):194–206
Engelhardt G, Michel D (1987) High-resolution solid-state NMR of silicates and zeolites. John Wiley & Sons, New York, NY
Fernández-Jiménez A, de la Torre AG, Palomo A, López-Olmo G, Alonso MM, Aranda MAG (2006) Quantitative determination of phases in the alkaline activation of fly ash. Part II: degree of reaction. Fuel 85(14–15):1960–1969
Duxson P, Provis JL, Lukey GC, Separovic F, Van Deventer JSJ (2005) 29Si NMR study of structural ordering in aluminosilicate geopolymer gels. Langmuir 21(7):3028–3036
Mackenzie K, Smith M (2002) Multinuclear solid-state NMR of inorganic materials. Pergamon materials series, vol 6, 1st ed. Oxford, Pergamon
Walkley B, Provis JL (2019) Solid-state nuclear magnetic resonance spectroscopy of cements. Mater Today Adv 1:100007
Zheng L, Wang W, Gao X (2016) Solidification and immobilization of MSWI fly ash through aluminate geopolymerization: Based on partial charge model analysis. Waste Manag 58:270–279
Trinh TT, Rozanska X, Delbecq F, Sautet P (2012) The initial step of silicate versusaluminosilicate formation in zeolite synthesis: a reaction mechanism in water with a tetrapropylammonium template. Phys Chem Chem Phys 14(10):3369–3380
Sagoe-Crentsil K, Weng L (2007) Dissolution processes, hydrolysis and condensation reactions during geopolymer synthesis: Part II. High Si/Al ratio systems. J Mater Sci 42(9):3007–3014
Fernández-Jiménez A, Palomo A, Sobrados I, Sanz J (2006) The role played by the reactive alumina content in the alkaline activation of fly ashes. Microporous Mesoporous Mater 91(1–3):111–119
Skibsted J, Snellings R (2019) Reactivity of supplementary cementitious materials (SCMs) in cement blends. Cem Concr Res 124:105799
Juenger MCG, Snellings R, Bernal SA (2019) Supplementary cementitious materials: New sources, characterization, and performance insights. Cem Concr Res 122:257–273
Acknowledgements
The authors would like to thank Gary Wenczel, Michael Davidson, and Yu-Han Yu in civil engineering department at University of Delaware at for their help to set up laboratory for our research group. The materials characterizations were carried out in Harker Interdisciplinary Science and Engineering laboratory at University of Delaware and with help from Gerald Poirier, Frank Kriss, Dr. Yong Zhao and Dr. Chaoying Ni in this laboratory. This study was funded through an NSF grant (No. #1538432) and through civil engineering department at University of Delaware.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Figure 829Si NMR spectra of empty NMR glass tube (a), glass tube filled with sodium silicate solution (b), and the sodium silicate solution (c) by subtracting spectra (a) from (b). The broad hump centered at −109.8 ppm seen in both the spectra (a) and (b) is attributed to signal from the glass tube. The peak at −72.6 ppm seen in the spectrum of the sodium silicate solution (c) indicates the aqueous Si is present as Q0, i.e., the Si monomers.
The solid-state MAS 27Al (shown in Fig. 9) and 23Na (shown in Fig. 10) NMR spectra of the phases that were removed by water during the washing process (i.e., residual phases by drying filtrates that were separated from the gel–water suspensions during washing). In the residue from suspension of the 3 mL gel in Fig. 9a, the Al mainly exists as aluminosilicate, as indicated by the sharp peak at 59.3 ppm. This presence of aluminosilicate is further confirmed by the peak at −4.1 ppm in the corresponding solid-state MAS 23Na NMR spectrum shown in Fig. 10a. To the contrary, in addition to the peak at around 56.6 ppm in the 27Al NMR spectrum, the spectrum of the residue from washing the 1 mL gel shows an evident at 3.9 ppm, which is attributed to a 6-coordianted peak. This observation is consistent with the strong peak at −0.5 ppm in the corresponding 23Na NMR spectrum shown in Fig. 10b, which is assigned to the free Na that is not bound to aluminosilicates. The presence of the strong 6-coordianted Al peak and the intense single form the free Na indicated that much of the Al has not participated in the reaction to form aluminosilicates when 1 mL of the NaOH solution was added during the gel synthesis, probably because the pH of 4.1 in the synthesizing mixtures was much lower than that is needed for geopolymerization.
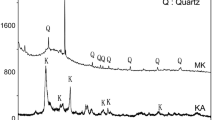
Figure 11 shows the SEM images using the LEI detector for the same samples as in Fig. 1 (with the SEI detector). The morphology of the flocculated particles in Fig. 11 is clearly revealed. While the sizes of the agglomerates increase with the increasing alkalinity as shown in Fig. 1, the flocculated particles (as elements that form the agglomerates) follow the same trend as shown here in Fig. 11. Specifically, while the size of the flocculated particles is too small to be discernible under such magnification (×20,000) for the 5 mL gel (Fig. 11a), it becomes larger for the 10 mL gel (Fig. 11b), and it increases further as circled in Fig. 11c for the 20 mL gel.
Rights and permissions
About this article
Cite this article
Chen, X., Mondal, P. Effects of NaOH amount on condensation mechanism to form aluminosilicate, case study of geopolymer gel synthesized via sol–gel method. J Sol-Gel Sci Technol 96, 589–603 (2020). https://doi.org/10.1007/s10971-020-05360-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-020-05360-6