Abstract
Radioiodine accumulates in aqueous solutions and off-gas streams during nuclear fuel reprocessing. In addition, radioiodine is highly mobile in geological environments. Most of the radioiodine can be captured during fuel reprocessing in off-gas streams using solid sorbents and scrubbing solutions. Once iodine is captured, it must be stored in a durable form for eventual disposal. Iodosodalite has been investigated as a waste form for radioiodine, however these synthesis processes often result in mixed products and iodine volatilization during consolidation. This paper proposes a novel approach to synthesizing iodosodalite utilizing a sol–gel method followed by heat treatment. This method was chosen to lower processing temperatures and improve product yield. Preliminary experiments conducted to determine the viability of this synthesis method are presented. In addition, consolidation of sol–gel derived iodosodalite with a glass binder was explored using three different methods: (1) incorporating the glass binder during gel preparation using alkoxide precursors; (2) separately preparing the glass binder using a sol–gel method; and (3) separately preparing the glass binder using a melt-quench technique. Glass-bonded iodosodalite was successfully synthesized using these novel sol–gel-based approaches.

Highlights
-
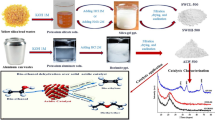
Iodosodalite precursors were produced with sol–gel approaches purely from alkoxides and NaI.
-
For B2, the NaBSi3O8 glass binder was added during the sol–gel process to produce the base gel.
-
For B3, the NaBSi3O8 glass binder was added in as alkoxides during the initial gel synthesis.
-
For B4, NaBSi3O8 glass binders were introduced as melt-quenched or sol–gel-derived additives.
-
Similar iodosodalite yields for samples with melt-quench and sol–gel-derived glass binders (B4).









Similar content being viewed by others
Change history
13 March 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10971-021-05477-2
References
Riley BJ, Vienna JD, Strachan DM, McCloy JS, Jerden Jr J (2016) Materials and processes for the effective capture and immobilization of radioiodine: a review. J Nucl Mater 470:307–326
Goldsmith JR, Grossman CM, Morton WE, Nussbaum RH, Kordysh EA, Quastel MR, Sobel RB, Nussbaum FD (1999) Juvenile hypothyroidism among two populations exposed to radioiodine. Environ Health Perspect 107:303–308
Grossman CM, Morton WE, Nussbaum RH (1996) Hypothyroidism and spontaneous abortions among Hanford, Washington, downwinders. Arch Environ Health 51:175–176
Grossman CM, Nussbaum RH, Nussbaum FD (2002) Thyrotoxicosis among Hanford, Washington, Downwinders: a community-based health survey. Arch Environ Health 57:9–15
Grossman CM, Nussbaum RH, Nussbaum FD (2003) Cancers among residents downwind of the Hanford, Washington, plutonium production site. Arch Environ Health 58:267–274
Thomas GD, Smith SM, Turcotte JA (2009) Using public relations strategies to prompt populations at risk to seek health information: the Hanford Community Health Project. Health Promotion Pract 10:92–101
Swift PN, Nutt WM (2012) Applying insights from repository safety assessments to evaluating impacts of partitioning and transmutation. 11th Actin Fission Prod Partition Transmut 145–154
OECD (2009) Mobile fission and activation products in nuclear waste disposal. NEA-6310 Organisation for Economic Co-Operation and Development, Nuclear Energy Agency, Paris, France
von Lensa W, Nabbi R, Rossbach M, Greneche D, Quiniou B, Boucher L, Delpech M, Gonzalez E, Alvarez F, Cunado MA, Serrano G, Cormenzana JL, Kuckshinrichs W, Odoj R, Wallenius J, Westlen D, Zimmerman C, Marivoet J (2008) RED-IMPACT: Impact of Partitioning, Transmutation, and Waste Reduction Technologies on the Final Nuclear Waste Disposal—Synthesis Report. Forschungszentrum Julich GmbH Zentralbibliothek, Verlag, Julich, GmbH
Chapman KW, Chupas PJ, Nenoff TM (2010) Radioactive iodine capture in silver-containing mordenites through nanoscale silver iodide formation. J Am Chem Soc 132:8897–8899
Haefner DR, Watson TL (2010) Summary of FY 2010 Iodine Capture Studies at the INL. Idaho National Laboratory, Idaho Falls, ID
Jubin RT (1980) Organic iodine removal from simulated dissolver off-gas streams using silver-exchanged mordenite. 16th DOE. Nucl Air Clean CONF 1:519–530
Jubin RT (1982) Organic iodine removal from simulated dissolver off-gas systems using partially exchanged silver mordenite. Nucl Air Clean 1:183–196
Matyáš J, Fryxell GE, Busche BJ, Wallace K, Fifield LS (2011) Functionalized silica aerogels: advanced materials to capture and immobilize radioactive iodine. Ceram Eng Sci 32:23–33
Matyáš J (2013) Silver-functionalized silica aerogels for iodine capture and immobilization. Separations and Waste Forms Research and Development FY 2012 Accomplishments Report
Riley BJ, Chun J, Um W, Lepry WC, Matyáš J, Olszta MJ, Li X, Polychronopoulou K, Kanatzidis MG (2013) Chalcogen-based aerogels as sorbents for radionuclide remediation. Environ Sci Technol 47:7540–7547
Riley BJ, Pierce D,A, Chun J, Matyáš J, Lepry WC, Garn T, Law J, Kanatzidis MG (2014) Polyacrylonitrile-chalcogel hybrid sorbents for radioiodine capture. Environ Sci Technol 48:5832–5839
Subrahmanyam KS, Sarma D, Malliakas CD, Polychronopoulou K, Riley BJ, Pierce DA, Chun J, Kanatzidis MG (2015) Chalcogenide aerogels as sorbents for radioactive iodine. Chem Mater 27:2619–2626
Ma S, Islam SM, Gu Q, Shim Y, Wang P, Li H, Sun G, Yang X, Kanatzidis MG (2014) Highly efficient iodine capture by layered double hydroxides intercalated with polysulfides. Adv Funct Mater 26:7114–7123
Park KS, Ni Z, Côté AP, Choi JY, Huang R, Uribe-Romo FJ, Chae HK, O’Keeffe M, Yaghi OM (2006) Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc Natl Acad Sci 103:10186–10191
Chui SS-Y, Lo SM-F, Charmant JPH, Orpen AG, Williams ID (1999) A chemically functionalizable nanoporous material [Cu3(TMA)2(H2O)3]n. Science 283:1148–1150
Trevorrow LE, Vandegrift GF, Kolba VM, Steindler MJ (1983) Compatibility of technologies with regulations in the waste management of H-3, I-129, C-14, and Kr-85. Part I. Initial information base. ANL-83-57 Argonne National Laboratory Argonne, IL
Collard GER, Hennart D, Van Dooren J, Goosens WRA (1980) Iodine trapping and conditioning in the Mercurex process. 16th DOE. Nucl Air Clean 552–564. https://inis.iaea.org/search/search.aspx?orig_q=RN:12639218
Stromatt R (1958) Removal of radioiodine from PUREX Off-gases with nitric acid and nitric acid-mercuric nitrate solutions. USAEC HW-55735 Hanford Atomic Products Operation, Richland, WA
Holladay DW (1979) A literature survey: methods for the removal of iodine species from off-gases and liquid waste streams of nuclear power and nuclear fuel reprocessing plants, with emphasis on solid sorbents. ORNL/TM-6350 Oak Ridge National Laboratory, Oak Ridge, TN
Mailen JC, Horner DE (1975) Removal of radioiodine from gas streams by electrolytic scrubbing. Nucl Technol 30:317–324
Horner DE, Mailen JC, Posey FA (1980) Electrolytic trapping of iodine from process gas streams, patent CA1086257A
Maddrell ER, Abraitis PK (2004) A comparison of wasteforms and processes for the immobilisation of iodine-129. Mater Res Soc 807:261–266
Dalba G, Fornasini P, Rocca F (1990) Short range order in AgI:Ag2O:B2O3 glasses: results from EXAFS and related techniques. J Non-Cryst Solids 123:310–314
Yang JH, Shin JM, Park JJ, Park G (2014) Waste form of silver iodide (AgI) with low-temperature sintering glasses. Sep Sci Technol 49:298–304
Mukunoki A, Chiba T, Suzuki Y, Uehara S, Asano H, Nishimura T (2007) Development of an iodine immobilization technique by low temperature vitrification with BiPbO2I. 11th Int Conf Environ Remed Radioact Waste Manag 459–464
Watanabe Y, Ikoma T, Yamada H, Stevens GW, Moriyoshi Y, Tanaka J, Komatsu Y (2010) Formation of hydroxyapatite nanocrystals on the surface of Ca-Al-layered double hydroxide. J Am Ceram Soc 93:1195–1200
Pierce EM, Mattigod SV, Westsik JH, Serne RJ, Icenhower JP, Scheele RD, Um W, Qafoku NP (2010) Review of potential candidate stabilization technologies for liquid and solid secondary waste streams. PNNL-19122, Pacific Northwest National Laboratory, Richland, WA
Audubert F, Carpena J, Lacout JL, Tetard F (1997) Elaboration of an iodine-bearing apatite Iodine diffusion into a Pb3(VO4)2 matrix. Solid State Ion 95:113–119
Campayo L, Grandjean A, Coulon A, Delorme R, Vantelon D, Laurencin D (2011) Incorporation of iodates into hydroxyapatites: a new approach for the confinement of radioactive iodine. J Mater Chem 21:17609–17611
Trill H, Eckert H, Srdanov VI (2003) Mixed halide sodalite solid solution systems. Hydrothermal synthesis and structural characterization by solid state NMR. J Phys Chem B 107:8779–8788
Strachan D, Babad H (1979) Iodide and iodate sodalites for the long-term storage of iodine-129. Rockwell International-Rockwell Hanford Operations, Richland, WA
Nakazawa T, Kato H, Okada K, Ueta S, Mihara M (2001) Iodine immobilization by sodalite waste form. Mater Res Soc 663:51–57
Maddrell E, Gandy A, Stennett M (2014) The durability of iodide sodalite. J Nucl Mater 449:168–172
Chong S, Peterson J, Nam J, Riley B, McCloy J (2017) Synthesis and characterization of iodosodalite. J Am Ceram Soc 100:2273–2284
Chong S, Peterson JA, Riley BJ, Tabada D, Wall D, Corkhill CL, McCloy JS (2018) Glass-bonded iodosodalite waste form for immobilization of 129I. J Nucl Mater 504:109–121
Nam J, Chong S, Riley BJ, McCloy JS (2018) Iodosodalite waste forms from low-temperature aqueous process. MRS Adv 3:1093–1103
Strachan DM, Babad H (1979) Iodide and iodate sodalites for long-term storage of I-129. Am Ceram Soc Bull 58:327
Riley BJ, Kroll JO, Pierce DA, Canfield NL, Williams BD, Snyder MV, Ebert WL, Frank SM (2015) Electrochemical waste form research: critical gap activities for fiscal year 2015. FCRD-JFCS-2015-20000706, PNNL-24662, Pacific Northwest National Laboratory, Richland, Washington
Riley BJ, Lepry WC, Crum JV (2016) Solution-derived sodalite made with Si- and Ge-ethoxide precursors for immobilizing electrorefiner salt. J Nucl Mater 468:140–146
Hench LL, West JK (1990) The sol–gel process. Chem Rev 90:33–72
Brinker CJ, Mukherjee SP (1981) Conversion of monolithic gels to glasses in a multicomponent silicate glass system. J Mater Sci 16:1980–1988
Yoldas BE (1977) Preparation of glasses and ceramics from metal-organic compounds. J Mater Sci 12:1203–1208
Mackenzie JD (1982) Glasses from melts and glasses from gels, A comparison. J Non-Cryst Solids 48:1–10
Vance ER, Davis J, Olufson K, Chironi I, Karatchevtseva I, Farnan I (2012) Candidate waste forms for immobilisation of waste chloride salt from pyroprocessing of spent nuclear fuel. J Nucl Mater 420:396–404
SRM-674b (2018) X-ray powder diffraction intensity set (Quantitative powder diffraction standard), National Institute of Standards and Technology
Cheary RW, Coelho AA, Cline JP (2004) Fundamental parameters line profile fitting in laboratory diffractometers. J Res Natl Inst Stand Technol 109:1–25
Williams BD, Neeway JJ, Snyder MMV, Bowden ME, Amonette JE, Arey BW, Pierce EM, Brown CF, Qafoku NP (2016) Mineral assemblage transformation of a metakaolin-based waste form after geopolymer encapsulation. J Nucl Mater 473:320–332
Thompson JG, Withers RL, Melnitchenko A, Palethorpe SR (1998) Cristobalite-related phases in the NaAlO2-NaAlSiO4 System. I. Two tetragonal and two orthorhombic structures. Acta Crystallogr Sect B 54:531–546
Acknowledgements
Pacific Northwest National Laboratory (PNNL) is operated by Battelle Memorial Institute for the DOE under contract DE-AC05-76RL01830. This work was performed under a project for the Nuclear Energy University Program funded by the Department of Energy Office of Nuclear Energy. Authors thank Ashutosh Goel and Saehwa Chong for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kroll, J.O., Riley, B.J., McCloy, J.S. et al. Sol–gel synthesis of iodosodalite precursors and subsequent consolidation with a glass binder made from oxides and sol–gel routes. J Sol-Gel Sci Technol 96, 564–575 (2020). https://doi.org/10.1007/s10971-020-05348-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-020-05348-2