Abstract
Transdermal delivery of drugs represents a non-invasive alternative treatment used not only for skin diseases. As one of the possible penetration enhancing agents, various types of nanoparticles (NPs) could be used. Silver NPs (AgNPs) could be used for some medical purposes considering their antibacterial and antiinflammatory properties. We demonstrate a novel method of quantification of permeated AgNPs, detection of AgNPs dissolving while passing the skin, and examination of interactions between skin and systems with AgNPs. Several AgNPs (exhibiting defined mean diameters of 20, 40, 60, and 100 nm) were added individually to the pure solvents commonly used in pharmaceuticals, namely ethanol, methanol, dimethyl sulfoxide, and demineralized water. AgNP dispersions in different solvents were applied to untreated samples of the skin. Attenuated total reflection technique was used for monitoring the kinetic series of infrared spectra to elucidate the time-dependent changes in the uppermost layer of the skin. The depth profiling spectra series were measured using confocal Raman microspectrometer. All recorded vibrational spectra were evaluated by multivariate statistical methods. A strong influence of AgNP size on the structural changes of the skin surface was evident. The largest changes of the skin structure were caused by the 20-nm and 40-nm AgNPs. Permeation of used AgNPs was studied on vertical Franz diffusion cells with detection of permeated AgNPs by new method based on single-particle inductively coupled plasma mass spectrometry.

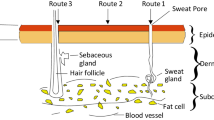
Graphical abstract








Similar content being viewed by others
Data availability
The data generated during the current study are available from the corresponding author on reasonable request.
References
Bakshi P, Jiang Y, Nakata T, Akaki J, Matsuoka N, Banga AK (2018) Formulation development and characterization of nanoemulsion-based formulation for topical delivery of heparinoid. J Pharm Sci 107:2883–2890
Baroli B, Ennas MG, Loffredo F, Isola M, Pinna R, Arturo López-Quintela M (2007) Penetration of metallic nanoparticles in human full-thickness skin. J Invest Dermatol 127:1701–1712
Bartosova L, Bajgar J (2012) Transdermal drug delivery in vitro using diffusion cells. Curr Med Chem 19:4671–4677
Danciu C, Pinzaru I, Coricovac D, Andrica F, Sizemore I, Dehelean C, Baderca F, Lazureanu V, Soica C, Mioc M, Radeke H (2019) Betulin silver nanoparticles qualify as efficient antimelanoma agents in in vitro and in vivo studies. Eur J Pharm Biopharm 134:1–19
Dragicevic N, Maibach H (2018) Combined use of nanocarriers and physical methods for percutaneous penetration enhancement. Adv Drug Deliv Rev 127:58–84
Escobar-Chávez JJ, Díaz-Torres R et al (2015) Nanocarriers for transdermal drug delivery. Res Rep Transderm Drug Deliv 4:23–33
Falamas A, Cinta-Pinzaru S et al (2010) Raman and FT-IR imaging of in-vivo damaged tissue induced by 7, 12-dimethylbenzanthracene (DMBA) in mouse models. Rom J Biophys 20:1–11
Friend DR (1992) In vitro skin permeation techniques. J Control Release 18:235–248
Gatto F, Bardi G (2018) Metallic nanoparticles: general research approaches to immunological characterization. Nanomaterials 8:753
Grodowska K, Parczewski A (2010) Organic solvents in the pharmaceutical industry. Acta Pol Pharm 67:3–12
Hineman A, Stephan C (2014) Effect of dwell time on single particle inductively coupled plasma mass spectrometry data acquisition quality. J Anal At Spectrom 29:1252–1257
Hwang K, Martin NE, Jiang L (2003) Permeation prediction of M100240 using the parallel artificial membrane permeability assay. J Pharm Pharmaceut Sci 6:315–320
Jeništová A, Dendisová M, Matějka P (2017) Study of plasmonic nanoparticles interactions with skin layers by vibrational spectroscopy. Eur J Pharm Biopharm 116:85–93
Jeništová A, Halajová L, Dendisová M, Matějka P (2018) Study of interactions between gallic acid and skin surface using infrared spectroscopy. Vib Spectrosc 97:119–128
Kraeling MEK, Topping VD, Keltner ZM, Belgrave KR, Bailey KD, Gao X, Yourick JJ (2018) In vitro percutaneous penetration of silver nanoparticles in pig and human skin. Regul Toxicol Pharmacol 95:314–322
Krukowski S, Karasiewicz M, Kolodziejski W (2017) Convenient UV-spectrophotometric determination of citrates in aqueous solutions with applications in the pharmaceutical analysis of oral electrolyte formulations. J Food Drug Anal 25:717–722
Laborda F, Bolea E, Jiménez-Lamana J (2014) Single particle inductively coupled plasma mass spectrometry: a powerful tool for nanoanalysis. Anal Chem 86:2270–2278
Larese FF, D'Agostin F et al (2009) Human skin penetration of silver nanoparticles through intact and damaged skin. Toxicology 255:3–37
Loula M, Kaňa A, Koplík R, Hanuš J, Vosmanská M, Mestek O (2019a) Analysis of silver nanoparticles using single-particle inductively coupled plasma – mass spectrometry (ICP-MS): parameters affecting the quality of results. Anal Lett 52:288–307
Loula M, Kaňa A, Mestek O (2019b) Non-spectral interferences in single-particle ICP-MS analysis: an underestimated phenomenon. Talanta 202:565–571
Marwah H, Garg T, Goyal AK, Rath G (2016) Permeation enhancer strategies in transdermal drug delivery. Drug Deliv 23:564–578
Meidan VM, Bonner MC, Michniak BB (2005) Transfollicular drug delivery - is it a reality? Int J Pharm 306:1–14
Mitrano DM, Lesher EK, Bednar A, Monserud J, Higgins CP, Ranville JF (2012) Detecting nanoparticulate silver using single-particle inductively coupled plasma–mass spectrometry. Environ Toxicol Chem 31:115–121
Neubert RH (2011) Potentials of new nanocarriers for dermal and transdermal drug delivery. Eur J Pharm Biopharm 7:1–2
Niska K, Zielinska E, Radomski MW, Inkielewicz-Stepniak I (2018) Metal nanoparticles in dermatology and cosmetology: interactions with human skin cells. Chem Biol Interact 295:38–51
Olesik JW, Gray PJ (2012) Considerations for measurement of individual nanoparticles or microparticles by ICP-MS: determination of the number of particles and the analyte mass in each particle. J Anal At Spectrom 27:1143–1155
Prow TW, Grice JE, Lin LL, Faye R, Butler M, Becker W, Wurm EMT, Yoong C, Robertson TA, Soyer HP, Roberts MS (2011) Nanoparticles and microparticles for skin drug delivery. Adv Drug Deliv Rev 63:470–491
Ruela ALM, Perissinato AG, Lino MES, Mudrik PS, Pereira GR (2016) Evaluation of skin absorption of drugs from topical and transdermal formulations. Braz J Pharm Sci 52:527–544
Samberg ME, Oldenburg SJ, Monteiro-Riviere NA (2010) Evaluation of silver nanoparticle toxicity in skin in vivo and keratinocytes in vitro. Environ Health Perspect 118:407–413
Tak YK, Pal S et al (2010) Shape-dependent skin penetration of silver nanoparticles: does it really matter? Sci Rep 5:16908–16919
Tfaili S, Gobinet C et al (2010) Confocal Raman microspectroscopy for skin characterization: a comparative study between human skin and pig skin. Analyst 137:3673–3682
Vogt A, Combadiere B, Hadam S, Stieler KM, Lademann J, Schaefer H, Autran B, Sterry W, Blume-Peytavi U (2006) 40 nm, but not 750 or 1500 nm, nanoparticles enter epidermal CD1a+ cells after transcutaneous application on human skin. J Invest Dermatol. 126:1316–1322
Vogt A, Rancan F, Ahlberg S, Nazemi B, Choe CS, Darvin ME, Hadam S, Blume-Peytavi U, Loza K, Diendorf J, Epple M, Graf C, Rühl E, Meinke MC, Lademann J (2014) Interaction of dermatologically relevant nanoparticles with skin cells and skin. Beilstein J Nanotechnol 5:2363–2373
Vuppugalla R, Chang SY, Zhang H, Marathe PH, Rodrigues DA (2007) Effect of commonly used organic solvents on the kinetics of cytochrome P450 2B6- and 2C8-dependent activity in human liver microsomes. Drug Metab Dispos 35:1990–1995
Code availability
Not applicable.
Funding
This study was funded by the project No. 17-00291S of Czech Science Foundation (GACR) the Operational Programme Prague – Competitiveness (CZ.2.16/3.1.00/24501), National Program of Sustainability (NPU I LO1613) MSMT-43760/2015, and from specific university research (MSMT No 21-SVV/2019).
Author information
Authors and Affiliations
Contributions
Adéla Jeništová conceptualized the study, and performed and analyzed the results (with Pavel Matějka cooperation) from vibrational spectroscopy studies. Martin Loula and Oto Mestek performed and analyzed the results from sp-ICP-MS method. Pavel Ulbrich contributed results from TEM. Pavel Matějka also reviewed and edited the original draft. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 907 kb)
Rights and permissions
About this article
Cite this article
Jeništová, A., Loula, M., Mestek, O. et al. The effect of silver nanoparticles on the penetration properties of the skin and quantification of their permeation through skin barrier. J Nanopart Res 22, 332 (2020). https://doi.org/10.1007/s11051-020-05061-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-020-05061-9