Abstract
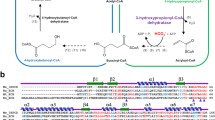
Here, we have analyzed the enzyme ornithine carbamoyltransferase (OCTase) in different classes of microorganisms belonging to psychrophiles, mesophiles and thermophiles. This OCTase catalyzes the formation of citrulline from carbamoyl phosphate (CP) and ornithine (ORN) in arginine biosynthesis pathway and has certain unique adaptations to regulate metabolic pathways in extreme conditions. The tertiary structure of OCTase showed two binding domains, the CP domain and ORN-binding domain at N and C terminals, respectively. We propose general acid–base catalysis in Pseudomonas gessardii between His259 and Asp220 in which later may act as a recipient of proton in the process. The comparative docking analysis showed that substrate-binding loops have been evolved to accommodate their lifestyles across the physiological temperature range where two substrates bind on two distinct loops in psychrophiles and mesophiles, whereas both the substrates bind on a single-substrate-binding loop in thermophiles and bring down the flexibility of the active site pocket to improve its evolutionary fitness.
Similar content being viewed by others
References
Berendsen HJC, van der Spoel D, van Drunen R (1995) GROMACS: a message-passing parallel molecular dynamics implementation. Comput Phys Commun 91:43–56
Canganella F, Wiegel J (2011) Extremophiles: From abyssal to terrestrial ecosystems and possibly beyond. Naturwissenschaften 98:253–279
Corpet F (1988) Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res 16:10881–10890
Davail S, Feller G, Narinx E, Gerday C (1994) Cold adaptation of proteins. Purification, characterization, and sequence of the heat-labile subtilisin from the Antarctic psychrophile Bacillus TA41. J Biol Chem 269:17448–17453
De Maayer P, Anderson D, Cary C, Cowan DA (2014) Some like it cold: understanding the survival strategies of psychrophiles. EMBO Rep 15:508–517
De Rivas B, Fox GC, Angulo I, Ripoll MM, Rodriguez H, Munoz R, Mancheno JM (2009) Crystal structure of the hexameric catabolic ornithine transcarbamylase from Lactobacillus hilgardii: structural insights into the oligomeric assembly and metal binding. J Mol Biol 393:425–434
Do CB, Katoh K (2009) Protein multiple sequence alignment. Functional Proteomics. Springer, London, pp 379–413
Gangwar P, Alam SI, Bansod S, Singh L (2009) Bacterial diversity of soil samples from the western Himalayas, India. Can J Microbiol 55:564–577
Gianese G, Bossa F, Pascarella S (2002) Comparative structural analysis of psychrophilic and meso- and thermophilic enzymes. Proteins Struct Funct Genet 47:236–249
Gouet P, Courcelle E, Stuart DI, Metoz F (2000) BIOINFORMATICS ESPript: analysis of multiple sequence alignments in PostScript. Bioinformatics 15:305–308
Gupta GN, Srivastava S, Khare SK, Prakash V (2014) Extremophiles: An Overview of Microorganism from Extreme Environment. Int J Agric Environ Biotechnol 7:371
Ha Y, McCann MT, Tuchman M, Allewell NM (1997) Substrate-induced conformational change in a trimeric ornithine transcarbamoylase. Proc Natl Acad Sci 94:9550–9555
Kim S, Thiessen PA, Bolton EE, Chen J, Fu G, Gindulyte A, Han L, He J, He S, Shoemaker BA, Wang J (2016) PubChem substance and compound databases. Nucleic Acids Res 44:D1202–D1213
Kohli U, Fatima Z, Hameed S (2013) Miraculous adaptations in extremophiles. Int J Appl Res Stud 2:1–9
Langley DB, Templeton MD, Fields BA, Mitchell RE, Collyer CA (2000) Mechanism of inactivation of ornithine transcarbamoylase by N δ-(N′-sulfodiaminophosphinyl)-l-ornithine, a true transition state analogue? Crystal structure and implications for catalytic mechanism. J Biol Chem 275:20012–20019
Laskowski RA, Swindells MB (2011) LigPlot + : multiple ligand à protein interaction diagrams for drug discovery. J Chem Inf Model 51:2778–2786
Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr 26:283–291
Legrain C, Halleux P, Stalon V, Glansdorff N (1972) The dual genetic control of ornithine carbamoyltransferase in escherichia coli a case of bacterial hybrid enzymes. Eur J Biochem 27:93–102
Martinez R, Schwaneberg U, Roccatano D (2011) Temperature effects on structure and dynamics of the psychrophilic protease subtilisin S41 and its thermostable mutants in solution. Protein Eng Des Sel 24:533–544
Mavromatis K, Tsigos I, Tzanodaskalaki M, Kokkinidis M, Bouriotis V (2002) Exploring the role of a glycine cluster in cold adaptation of an alkaline phosphatase. Eur J Biochem 269:2330–2335
McGuffin LJ, Bryson K, Jones DT (2000) The PSIPRED protein structure prediction server. Bioinformatics 16:404–405
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock4 and autoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30:2785–2791
Pulicherla KK, Ghosh M, Kumar PS, Rao KRSS (2011) Psychrozymes- the next generation industrial enzymes. Indust Enzy 1:1–7
Roovers M, Sanchez R, Legrain C, Glansdorff N (2001) Experimental evolution of enzyme temperature activity profile: Selection in vivo and characterization of low-temperature-adapted mutants of Pyrococcus furiosus ornithine carbamoyltransferase. J Bacteriol 183:1101–1105
Sankaranarayanan R, Cherney MM, Cherney LT, Garen CR, Moradian F, James MN (2008) The crystal structures of ornithine carbamoyltransferase from mycobacterium tuberculosis and its ternary complex with carbamoyl phosphate and l -norvaline reveal the enzyme’ s catalytic mechanism. J Mol Biol 375:1052–1063
Sarmiento F, Peralta R, Blamey JM (2015) Cold and hot extremozymes: industrial relevance and current trends. Front Bioeng Biotechnol 3:148
Schüttelkopf AW, Van Aalten DMF (2004) PRODRG: a tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr Sect D Biol Crystallogr 60:1355–1363
Shi D, Morizono H, Ha Y, Aoyagi M, Tuchman M, Allewell NM (1998) 1.85-Å resolution crystal structure of human ornithine transcarbamoylase complexed withn-phosphonacetyl-l-ornithine catalytic mechanism and correlation with inherited deficiency. J Biol Chem 273:34247–34254
Sundaresan R, Ebihara A, Kuramitsu S, Yokoyama S, Kumarevel T, Ponnuraj K (2015) Crystal structure analysis of ornithine transcarbamylase from Thermus thermophilus - HB8 provides insights on the plasticity of the active site. Biochem Biophys Res Commun 465:174–179
Tricot C, Villeret V, Sainz G, Dideberg O, Stalon V (1998) Allosteric regulation in Pseudomonas aeruginosa catabolic ornithine carbamoyltransferase revisited: association of concerted homotropic cooperative interactions and local heterotropic effects. J Mol Biol 283:695–704
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461
Villeret V, Clantin B, Tricot C, Legrain C, Roovers M, Stalon V, Beeumen GN (1998) The crystal structure of Pyrococcus furiosus ornithine carbamoyltransferase reveals a key role for oligomerization in enzyme stability at extremely high temperatures. Proc Natl Acad Sci 95:2801–2806
Xu Y, Feller G, Gerday C, Glansdorff N (2003) Metabolic enzymes from psychrophilic bacteria: challenge of adaptation to low temperatures in ornithine carbamoyltransferase from Moritella abyssi. J Bacteriol 185:2161–2168
Zhang Y (2008) I-TASSER server for protein 3D structure prediction. BMC Bioinform 9:1–8
Acknowledgements
This work was supported by the Science and Engineering Research Board-Department of Science and Technology, Govt. of India (PDF/2016/000818). YA lab is supported by Govt. of India extramural research funds from Department of Biotechnology and Indian Council of Medical Research (Ministry of Health & Family welfare, Govt. of India). Authors are thankful to the anonymous reviewers and the handling editor for their input on the initial version of the manuscript, it has hugely improved our work.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by S. Albers.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sharma, S., Sharma, S., Ahmed, M. et al. Ornithine carbamoyltransferase from psychrophiles to thermophiles: structural evolution of catalytic fold to accommodate physiological diversity. Extremophiles 25, 15–24 (2021). https://doi.org/10.1007/s00792-020-01208-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-020-01208-7