Abstract
Background
Measuring the mechanical properties of soft biological tissues in-vitro by using conventional methods (e.g. tension, compression, or indentation) is prone to external testing and sample preparation factors. For example, sample slippage affects the measurement accuracy of mechanical properties of soft biological tissues. In addition, samples have to go through a complex cutting process to be prepared in specific shapes, which increases the post-mortem time spent before testing.
Objective
The purpose of this study is to investigate the capability of a new technique to measure the mechanics of soft biological tissues without experiencing the conventional challenges. It measures the mechanical behavior of soft materials by introducing and expanding spherical cavity deformations within materials’ internal structure while generating two stresses simultaneously, radial and hoop stresses.
Methods
Two porcine livers were tested. The experimental data were used to calibrate the material parameters of three hyperelastic models, namely: Yeoh, Arruda-Boyce, and Ogden. Numerical simulations were performed to validate the material parameters. Also, Computed Tomography (CT) imaging technology was used to verify the configuration of the cavity deformations.
Results
The outcome of the numerical simulations showed that hyperelastic models were able to predict the material response to cavity loading. In addition, CT imaging confirmed the spherical configuration of the applied deformations.
Conclusions
From the experimental results, numerical simulations and the CT imaging, it can be concluded that the cavity expansion test is capable of measuring the mechanics of soft biological tissues without experiencing the difficulties encountered in conventional techniques.














Similar content being viewed by others
References
Krouskop TA, Wheeler TM, Kallel F, Garra BS, Hall T (1998) Elastic moduli of breast and prostate tissues under compression. Ultrason Imaging 20:260–274. https://doi.org/10.1177/016173469802000403
Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM (2005) Tensional homeostasis and the malignant phenotype. Cancer Cell 8:241–254. https://doi.org/10.1016/j.ccr.2005.08.010
Samani A, Plewes D (2007) An inverse problem solution for measuring the elastic modulus of intact ex vivo breast tissue tumours. Phys Med Biol 52:1247–1260. https://doi.org/10.1088/0031-9155/52/5/003
Yeh W-C, Li P-C, Jeng Y-M, Hsu HC, Kuo PL, Li ML, Yang PM, Lee PH (2002) Elastic modulus measurements of human liver and correlation with pathology. Ultrasound Med Biol 28:467–474. https://doi.org/10.1016/S0301-5629(02)00489-1
Last JA, Pan T, Ding Y, Reilly CM, Keller K, Acott TS, Fautsch MP, Murphy CJ, Russell P (2011) Elastic Modulus determination of Normal and glaucomatous human trabecular meshwork. Investig Opthalmology Vis Sci 52:2147–2152. https://doi.org/10.1167/iovs.10-6342
Eom J, Shi C, Xu XG, De S (2009) Modeling respiratory motion for cancer radiation therapy based on patient-specific 4DCT data. Med Image Comput Comput-Assist Interv MICCAI Int Conf Med Image Comput Comput-Assist Interv 12:348–355
Al-Mayah A, Moseley J, Brock KK (2008) Contact surface and material nonlinearity modeling of human lungs. Phys Med Biol 53:305–317. https://doi.org/10.1088/0031-9155/53/1/022
Al-Mayah A, Moseley J, Hunter S, Brock K (2015) Radiation dose response simulation for biomechanical-based deformable image registration of head and neck cancer treatment. Phys Med Biol 60:8481–8489. https://doi.org/10.1088/0031-9155/60/21/8481
Wittek A, Miller K, Kikinis R, Warfield SK (2007) Patient-specific model of brain deformation: application to medical image registration. J Biomech 40:919–929. https://doi.org/10.1016/j.jbiomech.2006.02.021
Cash DM, Miga MI, Glasgow SC, Dawant BM, Clements LW, Cao Z, Galloway RL, Chapman WC (2007) Concepts and preliminary data toward the realization of image-guided liver surgery. J Gastrointest Surg 11:844–859. https://doi.org/10.1007/s11605-007-0090-6
Al-Mayah A, Moseley J, Velec M, Brock K (2009) Deformable modeling of human liver with contact surface. In: 2009 IEEE Toronto international conference science and Technology for Humanity (TIC-STH). IEEE, Toronto, pp 137–140
Carter FJ, Frank TG, Davies PJ, McLean D, Cuschieri A (2001) Measurements and modelling of the compliance of human and porcine organs. Med Image Anal 5:231–236. https://doi.org/10.1016/S1361-8415(01)00048-2
Tay BK, Kim J, Srinivasan MA (2006) In vivo mechanical behavior of intra-abdominal organs. IEEE Trans Biomed Eng 53:2129–2138. https://doi.org/10.1109/TBME.2006.879474
Han L, Noble JA, Burcher M (2003) A novel ultrasound indentation system for measuring biomechanical properties of in vivo soft tissue. Ultrasound Med Biol 29:813–823. https://doi.org/10.1016/S0301-5629(02)00776-7
Fowlkes JB, Emelianov SY, Pipe JG, Skovoroda AR, Carson PL, Adler RS, Sarvazyan AP (1995) Magnetic-resonance imaging techniques for detection of elasticity variation. Med Phys 22:1771–1778. https://doi.org/10.1118/1.597633
Muthupillai R, Lomas D, Rossman P, Greenleaf J, Manduca A, Ehman R (1995) Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science 269:1854–1857. https://doi.org/10.1126/science.7569924
Nightingale K (2011) Acoustic radiation force impulse (ARFI) imaging: a review. Curr Med Imaging Rev 7:328–339. https://doi.org/10.2174/157340511798038657
Franchi-Abella S, Elie C, Correas J-M (2013) Ultrasound elastography: advantages, limitations and artefacts of the different techniques from a study on a phantom. Diagn Interv Imaging 94:497–501. https://doi.org/10.1016/j.diii.2013.01.024
Varghese T, Ophir J, Konofagou E, Kallel F, Righetti R (2001) Tradeoffs in Elastographic imaging. Ultrason Imaging 23:216–248. https://doi.org/10.1177/016173460102300402
Bouchard R, Dahl J, Hsu S, Palmeri M, Trahey G (2009) Image quality, tissue heating, and frame rate trade-offs in acoustic radiation force impulse imaging. IEEE Trans Ultrason Ferroelectr Freq Control 56:63–76. https://doi.org/10.1109/TUFFC.2009.1006
Kemper AR, Santago AC, Stitzel JD et al (2010) Biomechanical response of human liver in tensile loading. Ann Adv Automot Med Assoc Adv Automot Med Annu Sci Conf 54:15–26
Nguyễn NH, Dương MT, Trần TN, Phạm PT, Grottke O, Tolba R, Staat M (2012) Influence of a freeze–thaw cycle on the stress–stretch curves of tissues of porcine abdominal organs. J Biomech 45:2382–2386. https://doi.org/10.1016/j.jbiomech.2012.07.008
Dương MT, Nguyễn NH, Trần TN, Tolba RH, Staat M (2015) Influence of refrigerated storage on tensile mechanical properties of porcine liver and spleen. Int Biomech 2:79–88. https://doi.org/10.1080/23335432.2015.1049295
Lu Y-C, Untaroiu CD (2012) Freezing and decay effects on material properties of porcine kidney and liver. Biomed Sci Instrum 48:275–281
Brands DW, Bovendeerd PH, Peters GW, Wismans JS (2000) The large shear strain dynamic behaviour of in-vitro porcine brain tissue and a silicone gel model material. Stapp Car Crash J 44:249–260
Ng BH, Chou SM, Krishna V (2005) The influence of gripping techniques on the tensile properties of tendons. Proc Inst Mech Eng [H] 219:349–354. https://doi.org/10.1243/095441105X34239
Matthews GL, Keegan KG, Graham HL (1996) Effects of tendon grip technique (frozen versus unfrozen) on in vitro surface strain measurements of the equine deep digital flexor tendon. Am J Vet Res 57:111–115
Herzog W, Gal J (1999) Tendon. In: Biomechanics of musculo-skeletal system, 2nd edn. John Wiley, New York, pp 127–147
Nafo W, Al Mayah A (2018) Mechanical investigations of biological tissues using tensile loading and indentation. In: biomechanics of soft tissues, 1st ed. CRC Press, pp 27–54
Soden PD, Kershaw I (1974) Tensile testing of connective tissues. Med Biol Eng 12:510–518. https://doi.org/10.1007/BF02478609
Riemersa DJ, Schamhardt HC (1982) The cryo-jaw, a clamp designed for in vitro rheology studies of horse digital flexor tendons. J Biomech 15:619–620
Bergstrom J (2015) Mechanics of solid polymers: theory and computational modeling. Elsevier, Amsterdam
Nafo W, Al-Mayah A (2020) Characterization of PVA hydrogels’ hyperelastic properties by uniaxial tension and cavity expansion tests. Int J Non-Linear Mech 124:103515. https://doi.org/10.1016/j.ijnonlinmec.2020.103515
Nafo W, Al-Mayah A (2020) Mechanical characterization of PVA hydrogels’ rate-dependent response using multi-axial loading. PLoS One 15:e0233021. https://doi.org/10.1371/journal.pone.0233021
Barney CW, Dougan CE, McLeod KR et al (2020) Cavitation in soft matter. Proc Natl Acad Sci 117:9157–9165. https://doi.org/10.1073/pnas.1920168117
Chui C, Kobayashi E, Chen X, Hisada T, Sakuma I (2004) Combined compression and elongation experiments and non-linear modelling of liver tissue for surgical simulation. Med Biol Eng Comput 42:787–798. https://doi.org/10.1007/BF02345212
deBotton G, Bustamante R, Dorfmann A (2013) Axisymmetric bifurcations of thick spherical shells under inflation and compression. Int J Solids Struct 50:403–413. https://doi.org/10.1016/j.ijsolstr.2012.10.004
Yeoh OH (1993) Some forms of the strain energy function for rubber. Rubber Chem Technol 66:754–771. https://doi.org/10.5254/1.3538343
Arruda EM, Boyce MC (1993) A three-dimensional constitutive model for the large stretch behavior of rubber elastic materials. J Mech Phys Solids 41:389–412. https://doi.org/10.1016/0022-5096(93)90013-6
Ogden RW (1972) Large deformation isotropic elasticity - on the correlation of theory and experiment for incompressible rubberlike solids. Proc R Soc Math Phys Eng Sci 326:565–584. https://doi.org/10.1098/rspa.1972.0026
Shahzad M, Kamran A, Siddiqui MZ, Farhan M (2015) Mechanical characterization and FE Modelling of a Hyperelastic material. Mater Res 18:918–924. https://doi.org/10.1590/1516-1439.320414
Rashid B, Destrade M, Gilchrist MD (2014) Mechanical characterization of brain tissue in tension at dynamic strain rates. J Mech Behav Biomed Mater 33:43–54. https://doi.org/10.1016/j.jmbbm.2012.07.015
Zimberlin JA, McManus JJ, Crosby AJ (2010) Cavitation rheology of the vitreous: mechanical properties of biological tissue. Soft Matter 6:3632. https://doi.org/10.1039/b925407b
Zimberlin JA, Sanabria-DeLong N, Tew GN, Crosby AJ (2007) Cavitation rheology for soft materials. Soft Matter 3:763–767. https://doi.org/10.1039/b617050a
Brostow W, Pietkiewicz D, Wisner SR (2007) Polymer tribology in safety medical devices: retractable syringes. Adv Polym Technol 26:56–64. https://doi.org/10.1002/adv.20084
De Bardi M, Müller R, Grünzweig C et al (2018) Clogging in staked-in needle pre-filled syringes (SIN-PFS): influence of water vapor transmission through the needle shield. Eur J Pharm Biopharm 127:104–111. https://doi.org/10.1016/j.ejpb.2018.02.016
Zhang Q, Fassihi MA, Fassihi R (2018) Delivery Considerations of Highly Viscous Polymeric Fluids Mimicking Concentrated Biopharmaceuticals: Assessment of Injectability via Measurement of Total Work Done “WT.”. AAPS PharmSciTech 19:1520–1528. https://doi.org/10.1208/s12249-018-0963-x
Kasem H, Shriki H, Ganon L, Mizrahi M, Abd-Rbo K, Domb AJ (2019) Rubber plunger surface texturing for friction reduction in medical syringes. Friction 7:351–358. https://doi.org/10.1007/s40544-018-0227-5
volumegraphics.com. https://www.volumegraphics.com/. Accessed 3 Sep 2018
ABAQUS/CAE (2013) Abaqus/CAE v6.13 User’s Manual
Samur E, Sedef M, Basdogan C, Avtan L, Duzgun O (2007) A robotic indenter for minimally invasive measurement and characterization of soft tissue response. Med Image Anal 11:361–373. https://doi.org/10.1016/j.media.2007.04.001
Gao Z, Lister K, Desai JP (2010) Constitutive modeling of liver tissue: experiment and theory. Ann Biomed Eng 38:505–516. https://doi.org/10.1007/s10439-009-9812-0
Nafo W, Al-Mayah A (2019) Measuring Hyperelastic properties of hydrogels using cavity expansion method. Exp Mech 59:1047–1061. https://doi.org/10.1007/s11340-019-00504-4
Dương MT, Nguyễn NH, Trần TN, Tolba RH, Staat M (2015) Influence of refrigerated storage on tensile mechanical properties of porcine liver and spleen. Int Biomech 2:79–88. https://doi.org/10.1080/23335432.2015.1049295
Lu Y-C, Kemper AR, Untaroiu CD (2014) Effect of storage on tensile material properties of bovine liver. J Mech Behav Biomed Mater 29:339–349. https://doi.org/10.1016/j.jmbbm.2013.09.022
Tan Z, Dini D, Rodriguez y Baena F, Forte AE (2018) Composite hydrogel: a high fidelity soft tissue mimic for surgery. Mater Des 160:886–894. https://doi.org/10.1016/j.matdes.2018.10.018
Fu YB, Chui CK (2014) Modelling and simulation of porcine liver tissue indentation using finite element method and uniaxial stress–strain data. J Biomech 47:2430–2435. https://doi.org/10.1016/j.jbiomech.2014.04.009
Umale S, Chatelin S, Bourdet N, Deck C, Diana M, Dhumane P, Soler L, Marescaux J, Willinger R (2011) Experimental in vitro mechanical characterization of porcine Glisson’s capsule and hepatic veins. J Biomech 44:1678–1683. https://doi.org/10.1016/j.jbiomech.2011.03.029
Miller K, Chinzei K (1997) Constitutive modelling of brain tissue: experiment and theory. J Biomech 30:1115–1121. https://doi.org/10.1016/S0021-9290(97)00092-4
Nava A, Mazza E, Furrer M, Villiger P, Reinhart WH (2008) In vivo mechanical characterization of human liver. Med Image Anal 12:203–216. https://doi.org/10.1016/j.media.2007.10.001
Tan Z, Dini D, Rodriguez y Baena F, Forte AE (2018) Composite hydrogel: a high fidelity soft tissue mimic for surgery. Mater Des 160:886–894. https://doi.org/10.1016/j.matdes.2018.10.018
Wan WK, Campbell G, Zhang ZF, Hui AJ, Boughner DR (2002) Optimizing the tensile properties of polyvinyl alcohol hydrogel for the construction of a bioprosthetic heart valve stent. J Biomed Mater Res 63:854–861. https://doi.org/10.1002/jbm.10333
Roan E, Vemaganti K (2007) The nonlinear material properties of liver tissue determined from no-slip uniaxial compression experiments. J Biomech Eng 129:450–456. https://doi.org/10.1115/1.2720928
Chau KT (1997) Young’s Modulus interpreted from compression tests with end friction. J Eng Mech 123:1–7. https://doi.org/10.1061/(ASCE)0733-9399(1997)123:1(1)
Chen J, Patnaik SS, Prabhu RK, Priddy LB, Bouvard JL, Marin E, Horstemeyer MF, Liao J, Williams LN (2019) Mechanical response of porcine liver tissue under high strain rate compression. Bioengineering 6:49. https://doi.org/10.3390/bioengineering6020049
Al-Mayah A, Moseley J, Velec M et al (2010) Deformable image registration of heterogeneous human lung incorporating the bronchial tree: deformable image registration of heterogeneous human lung. Med Phys 37:4560–4571. https://doi.org/10.1118/1.3471020
Chui C, Kobayashi E, Chen X, Hisada T, Sakuma I (2007) Transversely isotropic properties of porcine liver tissue: experiments and constitutive modelling. Med Biol Eng Comput 45:99–106. https://doi.org/10.1007/s11517-006-0137-y
Li L, Maccabi A, Abiri A, Juo YY, Zhang W, Chang YJ, Saddik GN, Jin L, Grundfest WS, Dutson EP, Eldredge JD, Benharash P, Candler RN (2019) Characterization of perfused and sectioned liver tissue in a full indentation cycle using a visco-hyperelastic model. J Mech Behav Biomed Mater 90:591–603. https://doi.org/10.1016/j.jmbbm.2018.11.006
Miller K (2000) Constitutive modelling of abdominal organs. J Biomech 33:367–373. https://doi.org/10.1016/S0021-9290(99)00196-7
Chui C, Kobayashi E, Chen X, Hisada T, Sakuma I (2004) Combined compression and elongation experiments and non-linear modelling of liver tissue for surgical simulation. Med Biol Eng Comput 42:787–798. https://doi.org/10.1007/BF02345212
Marchesseau S, Chatelin S, Delingette H (2017) Nonlinear biomechanical model of the liver. In: Biomechanics of Living Organs. Elsevier, pp. 243–265
Chitnis GD, Verma MKS, Lamazouade J, Gonzalez-Andrades M, Yang K, Dergham A, Jones PA, Mead BE, Cruzat A, Tong Z, Martyn K, Solanki A, Landon-Brace N, Karp JM (2019) A resistance-sensing mechanical injector for the precise delivery of liquids to target tissue. Nat Biomed Eng 3:621–631. https://doi.org/10.1038/s41551-019-0350-2
DeCarlo PF, Slowik JG, Worsnop DR et al (2004) Particle morphology and density characterization by combined mobility and aerodynamic diameter measurements. Part 1: theory. Aerosol Sci Technol 38:1185–1205. https://doi.org/10.1080/027868290903907
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. In addition, the authors acknowledge that they did not receive financial support from any funding agencies to carry out the work.
Conflict of Interest
There are no conflicts to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1
Appendix 1
The definition of R is very critical to determine the deformation term in the circumferential direction, λ. In this work, R was estimated to be 1.42 mm; this radius is derived from an initial volume which is expressed as
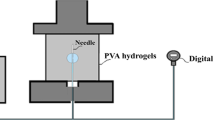
Where Vn-b is the volume of the balloon region which is 2.837 mm3, The dimensions of this region are described in Fig. 2 of the main manuscript; Vp is the volume that is introduced into the balloon before the tissues start to resist the cavity expansion, which is 9.177 mm3 as indicated by the pressure sensor data in Fig.7, see [72] for more details about the mechanism of injecting fluids into soft biological tissues. When the needle was inserted inside the tissues, it created a channel along the needle’s shaft. This channel made the tissues loose resulting into a negligible resistance against initially introduced volumes. A FE simulation was conducted for a process similar to that of a flat punch indentation test to calculate the measured initial volume (Vi) numerically. It was found that the magnitude of the volume induced due to a displacement equal to the thickness of the needle-balloon region was around 12 mm3, similar to the experimental Vi. This indicates that the insertion of the needle created a cut that allows the tissues to displace during initial volumes of inflation before resisting expansion.
Although, CT images have shown that the inflated balloon was nearly spherical in the injected volumes, the initial volume of the balloon has a cylindrical configuration. However, the stretch ratio (λ) can be calculated based on the volumetric ratio. Therefore, the exact geometry of the cavity does not affect the stretch (λ) calculation. The initial cylindrical volume can be represented by an equivalent spherical volume by using the concept of Equivalent-Volume Diameter (EVD) which was adopted in this study to define the initial radius, see [73] for more details about EVD. The EVD is based on equating irregular-shaped volumes to an equivalent sphere volume with a diameter of
R is then calculated as \( \frac{{\mathrm{D}}_{\mathrm{e}}}{2} \)
Rights and permissions
About this article
Cite this article
Nafo, W., Al-Mayah, A. Measuring the Hyperelastic Response of Porcine Liver Tissues In-Vitro Using Controlled Cavitation Rheology. Exp Mech 61, 445–458 (2021). https://doi.org/10.1007/s11340-020-00674-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11340-020-00674-6