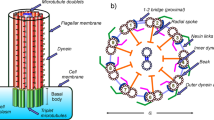
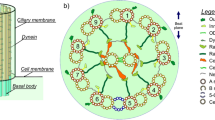
Abstract
To adapt to changing environments cells must signal and signaling requires messengers whose concentration varies with time in space. We here consider the messenger role of calcium ions implicated in regulation of the wave-like bending dynamics of cilia and flagella. The emphasis is on microtubules as polyelectrolytes serving as transmission lines for the flow of Ca2+ signals in the axoneme. This signaling is superimposed with a geometric clutch mechanism for the regulation of flagella bending dynamics and our modeling produces results in agreement with experimental data.
Similar content being viewed by others
References
Bannai H, Yoshimura M, Takahashi K, Shingyoji C (2000) Calcium regulation of microtubule sliding in reactivated sea urchin sperm flagella. J Cell Sci 113:831–839
Bayly PV, Wilson KS (2014) Equations of interdoublet separation during flagella motion reveal mechanisms of wave propagation and instability. Biophys J 107:1756–1772
Berridge MJ, Bootman MD, Roderick HL (2003) Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4:517–529
Brokaw CJ (2002) Computer simulations of flagellar movement viii: coordination of dynein by local curvature control can generate helical bending waves. Cell Motil Cytoskeleton 53:103–124
Brokaw CJ (2014) Computer simulation of flagellar movement X: doublet pair splitting and bend propagation modeled using stochastic dynein kinetics. Cytoskeleton 71:273–284
Burgoyne RD (2007) Neuronal calcium sensor proteins: generating diversity in neuronal Ca2+signalling. Nat Rev Neurosci 8:182–193
Cantero MDR, Perez PL, Scarinci N, Cantiello HF (2019) Two-dimensional brain microtubule structures behave as memristive devices. Sci Rep 9:12398
Clapham DE (2007) Calcium signaling. Cell 131:1047–1058
DeCaen PG, Delling M, Vien TN, Clapham DE (2013) Direct recording and molecular identification of the calcium channel of primary cilia. Nature 504:315–318
Doerner JF, Delling M, Clapham DE (2015) Ion channels and calcium signaling in motile cilia. eLife 4: e11066.
Frieden BR, Gatenby RA (2019) Signal transmission through elements of cytoskeleton from an optimized information network in eucariotic cells. Sci Rep 9:6110
Friesen DE, Craddock TJA, Priel A, Tuszynski JA (2014) Fields of the Cell, p243–265
Gadêlha H, Hernández-Herrera P, Montoya F, Darszon A, Corkidi G (2020) Human sperm uses asymmetric and anisotropic flagellar controls to regulate swimming symmetry and cell steering. Sci Adv 6:31, eaba5168
Hilfinger A, Chattopadyay AK, Jülicher F (2009) Nonlinear dynamics of cilia and flagella. Phys Rev E 79:051918
Hines M, Blum JJ (1978) Bend propagation in flagella. I. Derivation of equations of motion and their simulation. Biophys J 23:41–57
Howard J (2009) Mechanical signaling in networks of motor and cytoskeletal proteins. Annu Rev Biophys 38:217–234
Hunley C, Uribe D, Marucho M (2018) A multi-scale approach to describe electrical impulses propagating along actin filaments in both intracellular and in vitro conditions. RSC Adv 8:12017–12028
Ikegami K, Sato S, Nakamura K, Ostrowski LE, Setou M (2010) Tubulin polyglutamylation is essential for airway ciliary function through the regulation of beating asymmetry. PNAS 107:10490–10495
Kalra A, Patel S, Bhuiyan A, Preto J, Scheuer K, Mohammed U, Lewis J, Rezania V, Shankar K, Tuszynski JA (2019) On the capacitive properties of individual microtubules and their meshworks. arXiv:1905.02865
Kikkawa M (2013) Big steps toward understanding dynein. J Cell Biol 202(1):15–23
Lin J, Nicastro D (2018) Asymmetric distribution and spatial switching of dynein activity generates ciliary motility. Science 360:3687
Lindeman CB (2002) Geometric clutch Model Version 3: The role of the inner and outer arm dyneins in the ciliary beat. Cell Mot Cytoskel 52:242–254
Lindeman C (2007) The geometric clutch as a working hypothesis for future research on cilia and flagella. Ann N Y Acad Sci 1101:477–493
Manning GS (2008) Approximate solutions to some problems in polyelectrolyte theory involving nonuniform charge distributions. Macromolecules 41:6217–6227
Manning GS (2011) A counterion condensation theory for the relaxation, rise and frequency dependence of the parallel polarization of rodlike polyelectrolytes. Eur Phys J E 34:1–7
Minoura J, Muto E (2006) Dielectric measurement of individual microtubules using the electroorientation method. Biophys J 90:3739–3748
Nicastro D, Schwartz C, Pierson J, Gaudette R, Porter ME, McIntosh JR (2006) The molecular architecture of axonemes revealed by cryoelectron tomography. Science 313:944–948
Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N (1998) Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 95:829–837
Poznanski RR, Cacha LA, Al-Wesabi YMS, Ali J, Bahadoran M, Yupapin PP, Yunus J (2017) Erratum: Solitonic conduction of electrotonic signals in neuronal branchlets with polarized microstructure. Sci Rep 7:2746
Priel A, Ramos AJ, Tuszynski JA, Cantiello HF (2006) A biopolymer transistor: electrical amplification by microtubules. Biophys J 90:4639–4643
Priel A, Ramos AJ, Tuszynski JA, Cantiello HF (2008) Effect of calcium on electrical energy transfer by microtubules. J Biol Phys 34:475–485
Riedel-Kruse IH, Hilfinger A, Howard J, Jϋlicher F (2007) How molecular motors shape the flagellar beat. HFSP J 1:192–208
Sakato M, King SM (2003) Calcium regulates atp-sensitive microtubule binding by Chlamydomonas outer arm dynein. JBC 278:43571–43579
Sakato M, Sakakibara H, King SM (2007) Chlamydomonas outer arm dynein alters conformation in response to Ca2+. Mol Biol Cell 18:3620–3634
Santelices IB, Friesen DE, Bell C, Hough CM, Xiao J, Kalra A, Kar P, Freedman H, Rezania V, Lewis JD, Shankar K, Tuszynski JA (2017) Response to alternating electric fields of tubulin dimers and microtubule ensemles in electrolytic solutions. Sci Rep 7:9594
Sartori P (2015) Effect of curvature and normal forces on motor regulation of cilia. PhD Thesis, TechnischeUniversität Dresden, Germany
Sartori P, Geyer V, Scholich A, Jülicher F, Howard J (2016) Dynamic curvature regulation accounts for the symmetric and asymmetric beats of Chlamydomonas flagella. eLife 5:e13258
Sartori P, Geyer V, Scholich A, Jϋlicher F, Howard J (2016) Dynamic curvature regulation accounts for the symmetric and asymmetric beats of Chlamydomonas flagella. eLife 5:e13258
Satarić MV, Ilić DI, Ralević N, Tuszynski JA (2009) A nonlinear model of ionic wave propagation along microtubules. Eur Biophys J 38:637–647
Satarić MV, Nemeš T, Satarić BM, Sekulić D, Zdravković S (2020) Calcium ions tune the beats of cilia and flagella. BioSystems 196:104172
Satarić MV, Nemeš T, Sekulić D, Tuszynski JA (2019) How signals of calcium ions initiate the beats of cilia and flagella. BioSystems 182:42–51
Satarić MV, Sekulić D, Živanov M (2010) Solitonic ionic currents along microtubules. J Comput Theor Nanosci 7:1–10
Sekulić DL, Satarić BM, Tuszynski JA, Satarić MV (2011) Nonlinear ionic pulses along microtubules. Eur Phys J E 34:45–49
Sekulić DL, Satarić BM, Zdravković S, Bugay AN, Satarić MV (2016) Nonlinear dynamics of c-terminal tails in cellular microtubules. Chaos 26:073119
Shen C, Guo W (2018) Ion permeability of a microtubule in neuron environment. J Phys Chem Lett 9:2009–2014
Siwy ZS, Powell MR, Petrov A, Kalman E, Trautman C, Eisenberg RS (2006) Calcium induced voltage gating in single conical nanopores. Nano Lett 8:1729–1734
Sleigh MA, Blake JR, Liron N (1988) The propulsion of mucus by cilia. Am Rev Respir Dis 137:726–741
Smith EF (2002) Regulation of flagellar dynein by calcium and a role of an axonemal calmodulin dependent kinase. Mol Biol Cell 13:3303–3313
Strynadka NC, James MN (1989) Crystal structure of the helix-loop-helix calcium-binding proteins. Annu Rev Biochem 58:951–998
Tuszynski JA, Friesen D, Freedman H, Sbitnev VI, Kim H, Santelices I, Kalra AP, Patel SD, Shankar K, Chua LO (2020) Microtubules as sub-cellular memristors. Sci Rep 10:2108
Tuszynski JA, Satarić MV, Sekulić DL, Satarić BM, Zdravković S (2018) Nonlinear calcium ion waves along actin filaments control active hair-bundle motility. BioSystems 173:181–190
Vernon G, Woolley DM (2004) Basal sliding and the mechanics of oscillation in a mammalian sperm flagellum. Biophys J 87:3934–3944
Witman GB (2009) The Chlamydomonas Sourcebook; Cell Motility and Behaviour. Volume 3. Second ed., Academic Press
Acknowledgements
This research was financially supported by the Provincial Secretariat for Higher Education and Scientific Research of AP Vojvodina (Project No. 1144512708/201603), also by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Project No. OI171009, III43008, and III45010) and by project of Serbian Academy of Sciences and Arts.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1
Finding the second derivative in Eq. (3.32).
Appendix 2
We start with the dimensionless variable \(\xi \)
so that Eq. (3.30) now reads:
Using the abbreviations for \({C}_{1}\left(\sigma \right)\), \({C}_{2}\left(\sigma \right)\), Eqs. (3.34, 3.35) we go over to new ones \({\lambda }_{1}\), \({\lambda }_{2}\) and \(\gamma \) as expressed in the set of Eqs. (3.38, 3.39, 3.40). We just illustrate the specified numerical calculation for parameter:
Similarly, we completed the explicit form of Eq. (3.41).
Rights and permissions
About this article
Cite this article
Satarić, M.V., Zdravković, S., Nemeš, T. et al. Calcium signaling modulates the dynamics of cilia and flagella. Eur Biophys J 49, 619–631 (2020). https://doi.org/10.1007/s00249-020-01471-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-020-01471-8