Abstract
Background
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive human malignancies. Cell-cycle-related and expression-elevated protein in tumor (CREPT) plays an important role in the phosphorylation of RNA Pol II, and has been implicated in the development of several types of cancer. As yet, however, there have been no reports on its role in PDAC. Here, we aimed to explore the value of CREPT as a prognostic biomarker in PDAC.
Methods
CREPT expression was assessed by immunohistochemistry (IHC) on a tissue microarray containing samples from 375 PDAC patients. Kaplan-Meier and Cox regression analyses were performed to explore the independent prognostic value of CREPT expression for the disease-free survival (DFS) and overall survival (OS) of PDAC patients. A Cell Counting Kit-8 (CCK8) assay was used to determine the growth rates and gemcitabine sensitivities of PDAC cells, while a Transwell assay was used to determine the migration and invasion abilities of PDAC cells. Subcutaneous xenografts were used to explore the effect of CREPT expression on tumor growth in vivo.
Results
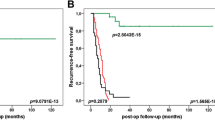
We found that CREPT is highly expressed in tumor tissues and may serve as an independent prognostic biomarker for DFS and OS of PDAC patients. In vitro assays revealed that CREPT expression promotes the proliferation, migration, invasion and gemcitabine resistance of PDAC cells, and in vivo assays showed that CREPT expression knockdown led to inhibition of PDAC tumor growth.
Conclusions
We conclude that high CREPT expression enhances the proliferation, migration, invasion and gemcitabine resistance of PDAC cells. In addition, we conclude that CREPT may serve as an independent prognostic biomarker and therapeutic target for PDAC patients.




Similar content being viewed by others
Abbreviations
- CCK8:
-
Cell Counting Kit-8
- CES:
-
composite expression score
- CI:
-
confidence interval
- CREPT:
-
cell-cycle-related and expression-elevated protein in tumor
- CTD:
-
C-terminal domain
- DFS:
-
disease-free survival
- HE:
-
Hematoxylin-eosin
- IHC:
-
immunohistochemistry
- OS:
-
overall survival
- PDAC:
-
pancreatic ductal adenocarcinoma
- RPRD1B:
-
regulation of the nuclear precursor RNA domain containing 1B
- SDS-PAGE:
-
SDS-polyacrylamide gel electrophoresis
- TMA:
-
tissue microarray
References
R.L. Siegel, K.D. Miller, A. Jemal, Cancer statistics, 2019. CA Cancer J Clin 69, 7–34 (2019)
M. Hidalgo, Pancreatic cancer. N Engl J Med 362, 1605–1617 (2010)
S. Maeda, M. Unno, J. Yu, Adjuvant and neoadjuvant therapy for pancreatic cancer. J Pancreatol 2, 100–106 (2019)
D. Li, K. Xie, R. Wolff, J.L. Abbruzzese, Pancreatic cancer. Lancet 363, 1049–1057 (2004)
A. Fesler, J. Ju, Development of microRNA-based therapy for pancreatic cancer. J Pancreatol 2, 147–151 (2019)
M.A. Tempero, M.P. Malafa, M. Al-Hawary, H. Asbun, A. Bain, S.W. Behrman, A.B. Benson 3rd, E. Binder, D.B. Cardin, C. Cha, E.G. Chiorean, V. Chung, B. Czito, M. Dillhoff, E. Dotan, C.R. Ferrone, J. Hardacre, W.G. Hawkins, J. Herman, A.H. Ko, S. Komanduri, A. Koong, N. LoConte, A.M. Lowy, C. Moravek, E.K. Nakakura, E.M. O'Reilly, J. Obando, S. Reddy, C. Scaife, S. Thayer, C.D. Weekes, R.A. Wolff, B.M. Wolpin, J. Burns, S. Darlow, Pancreatic adenocarcinoma, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 15, 1028–1061 (2017)
G. Ohshio, T. Manabe, Y. Watanabe, K. Endo, H. Kudo, T. Suzuki, T. Tobe, Comparative studies of DU-PAN-2, carcinoembryonic antigen, and CA19-9 in the serum and bile of patients with pancreatic and biliary tract diseases: Evaluation of the influence of obstructive jaundice. Am J Gastroenterol 85, 1370–1376 (1990)
N. Duraker, S. Hot, Y. Polat, A. Höbek, N. Gençler, N. Urhan, CEA, CA 19-9, and CA 125 in the differential diagnosis of benign and malignant pancreatic diseases with or without jaundice. J Surg Oncol 95, 142–147 (2007)
D. Lu, Y. Wu, Y. Wang, F. Ren, D. Wang, F. Su, Y. Zhang, X. Yang, G. Jin, X. Hao, D. He, Y. Zhai, D.M. Irwin, J. Hu, J.J. Sung, J. Yu, B. Jia, Z. Chang, CREPT accelerates tumorigenesis by regulating the transcription of cell-cycle-related genes. Cancer Cell 21, 92–104 (2012)
G. Pineda, Z. Shen, C.P. de Albuquerque, E. Reynoso, J. Chen, C.C. Tu, W. Tang, S. Briggs, H. Zhou, J.Y. Wang, Proteomics studies of the interactome of RNA polymerase II C-terminal repeated domain. BMC Res Notes 8, 616 (2015)
S. Egloff, M. Dienstbier, S. Murphy, Updating the RNA polymerase CTD code: Adding gene-specific layers. Trends Genet 28, 333–341 (2012)
R.J. Sims 3rd, L.A. Rojas, D.B. Beck, R. Bonasio, R. Schuller, W.J. Drury 3rd, D. Eick, D. Reinberg, The C-terminal domain of RNA polymerase II is modified by site-specific methylation. Science 332, 99–103 (2011)
Z. Ni, J.B. Olsen, X. Guo, G. Zhong, E.D. Ruan, E. Marcon, P. Young, H. Guo, J. Li, J. Moffat, A. Emili, J.F. Greenblatt, Control of the RNA polymerase II phosphorylation state in promoter regions by CTD interaction domain-containing proteins RPRD1A and RPRD1B. Transcription 2, 237–242 (2011)
Y. Wang, H. Qiu, W. Hu, S. Li, J. Yu, RPRD1B promotes tumor growth by accelerating the cell cycle in endometrial cancer. Oncol Rep 31, 1389–1395 (2014)
Y. She, J. Liang, L. Chen, Y. Qiu, N. Liu, X. Zhao, X. Huang, Y. Wang, F. Ren, Z. Chang, P. Li, CREPT expression correlates with poor prognosis in patients with retroperitoneal leiomyosarcoma. Int J Clin Exp Pathol 7, 6596–6605 (2014)
G. Yang, G. Xiong, M. Feng, F. Zhao, J. Qiu, Y. Liu, Z. Cao, H. Wang, J. Yang, L. You, L. Zheng, T. Zhang, Y. Zhao, OLR1 promotes pancreatic Cancer metastasis via increased c-Myc expression and transcription of HMGA2. Mol Cancer Res 18, 685–697 (2020)
C. Osborne, P. Wilson, D. Tripathy, Oncogenes and tumor suppressor genes in breast cancer: Potential diagnostic and therapeutic applications. Oncologist 9, 361–377 (2004)
J. Liu, H. Liu, X. Zhang, P. Gao, J. Wang, Z. Hu, Identification and characterization of P15RS, a novel P15(INK4b) related gene on G1/S progression. Biochem Biophys Res Commun 299, 880–885 (2002)
P. Komarnitsky, E.J. Cho, S. Buratowski, Different phosphorylated forms of RNA polymerase II and associated mRNA processing factors during transcription. Genes Dev 14, 2452–2460 (2000)
B.N. Devaiah, B.A. Lewis, N. Cherman, M.C. Hewitt, B.K. Albrecht, P.G. Robey, K. Ozato, R.J. Sims 3rd, D.S. Singer, BRD4 is an atypical kinase that phosphorylates serine2 of the RNA polymerase II carboxy-terminal domain. Proc Natl Acad Sci U S A 109, 6927–6932 (2012)
B. Bartkowiak, P. Liu, H.P. Phatnani, N.J. Fuda, J.J. Cooper, D.H. Price, K. Adelman, J.T. Lis, A.L. Greenleaf, CDK12 is a transcription elongation-associated CTD kinase, the metazoan ortholog of yeast Ctk1. Genes Dev 24, 2303–2316 (2010)
S. Buratowski, Progression through the RNA polymerase II CTD cycle. Mol Cell 36, 541–546 (2009)
B.M. Peterlin, D.H. Price, Controlling the elongation phase of transcription with P-TEFb. Mol Cell 23, 297–305 (2006)
A.L. Mosley, S.G. Pattenden, M. Carey, S. Venkatesh, J.M. Gilmore, L. Florens, J.L. Workman, M.P. Washburn, Rtr1 is a CTD phosphatase that regulates RNA polymerase II during the transition from serine 5 to serine 2 phosphorylation. Mol Cell 34, 168–178 (2009)
S. Egloff, J. Zaborowska, C. Laitem, T. Kiss, S. Murphy, Ser7 phosphorylation of the CTD recruits the RPAP2 Ser5 phosphatase to snRNA genes. Mol Cell 45, 111–122 (2012)
A.R. Bataille, C. Jeronimo, P.E. Jacques, L. Laramee, M.E. Fortin, A. Forest, M. Bergeron, S.D. Hanes, F. Robert, A universal RNA polymerase II CTD cycle is orchestrated by complex interplays between kinase, phosphatase, and isomerase enzymes along genes. Mol Cell 45, 158–170 (2012)
S. Hausmann, H. Koiwa, S. Krishnamurthy, M. Hampsey, S. Shuman, Different strategies for carboxyl-terminal domain (CTD) recognition by serine 5-specific CTD phosphatases. J Biol Chem 280, 37681–37688 (2005)
S. Krishnamurthy, X. He, M. Reyes-Reyes, C. Moore, M. Hampsey, Ssu72 is an RNA polymerase II CTD phosphatase. Mol Cell 14, 387–394 (2004)
S. Egloff, S. Murphy, Cracking the RNA polymerase II CTD code. Trends Genet 24, 280–288 (2008)
W. Kong, K. Engel, J. Wang, Mammalian nucleoside transporters. Curr Drug Metab 5, 63–84 (2004)
J.P. Hsin, A. Sheth, J.L. Manley, RNAP II CTD phosphorylated on threonine-4 is required for histone mRNA 3′ end processing. Science 334, 683–686 (2011)
A. Mayer, M. Heidemann, M. Lidschreiber, A. Schreieck, M. Sun, C. Hintermair, E. Kremmer, D. Eick, P. Cramer, CTD tyrosine phosphorylation impairs termination factor recruitment to RNA polymerase II. Science 336, 1723–1725 (2012)
B.M. Lunde, S.L. Reichow, M. Kim, H. Suh, T.C. Leeper, F. Yang, H. Mutschler, S. Buratowski, A. Meinhart, G. Varani, Cooperative interaction of transcription termination factors with the RNA polymerase II C-terminal domain. Nat Struct Mol Biol 17, 1195–1201 (2010)
K. Mei, Z. Jin, F. Ren, Y. Wang, Z. Chang, X. Wang, Structural basis for the recognition of RNA polymerase II C-terminal domain by CREPT and p15RS. Sci China Life Sci 57, 97–106 (2014)
Y. Wu, Y. Zhang, H. Zhang, X. Yang, Y. Wang, F. Ren, H. Liu, Y. Zhai, B. Jia, J. Yu, Z. Chang, p15RS attenuates Wnt/{beta}-catenin signaling by disrupting {beta}-catenin.TCF4 interaction. J Biol Chem 285, 34621–34631 (2010)
E.A. Klein, R.K. Assoian, Transcriptional regulation of the cyclin D1 gene at a glance. J Cell Sci 121, 3853–3857 (2008)
M. Shtutman, J. Zhurinsky, I. Simcha, C. Albanese, M. D'Amico, R. Pestell, A. Ben-Ze'ev, The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A 96, 5522–5527 (1999)
I. Matsumura, T. Kitamura, H. Wakao, H. Tanaka, K. Hashimoto, C. Albanese, J. Downward, R.G. Pestell, Y. Kanakura, Transcriptional regulation of the cyclin D1 promoter by STAT5: Its involvement in cytokine-dependent growth of hematopoietic cells. EMBO J 18, 1367–1377 (1999)
D.C. Guttridge, C. Albanese, J.Y. Reuther, R.G. Pestell, A.S. Baldwin Jr., NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol 19, 5785–5799 (1999)
R.A. Cavallo, R.T. Cox, M.M. Moline, J. Roose, G.A. Polevoy, H. Clevers, M. Peifer, A. Bejsovec, Drosophila Tcf and Groucho interact to repress wingless signalling activity. Nature 395, 604–608 (1998)
H. Clevers, R. Nusse, Wnt/beta-catenin signaling and disease. Cell 149, 1192–1205 (2012)
J.N. Anastas, R.T. Moon, WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer 13, 11–26 (2013)
D. Ma, Y. Zou, Y. Chu, Z. Liu, G. Liu, J. Chu, M. Li, J. Wang, S.Y. Sun, Z. Chang, A cell-permeable peptide-based PROTAC against the oncoprotein CREPT proficiently inhibits pancreatic cancer. Theranostics 10, 3708–3721 (2020)
C.R. Ferrone, M.W. Kattan, J.S. Tomlinson, S.P. Thayer, M.F. Brennan, A.L. Warshaw, Validation of a postresection pancreatic adenocarcinoma nomogram for disease-specific survival. J Clin Oncol 23, 7529–7535 (2005)
Z. Zheng, M. Wang, C. Tan, Y. Chen, J. Ping, R. Wang, X. Liu, Disparities in survival by stage after surgery between pancreatic head and body/tail in patients with nonmetastatic pancreatic cancer. PLoS One 14, e0226726 (2019)
L.K. Winer, V.K. Dhar, K. Wima, M.C. Morris, T.C. Lee, S.A. Shah, S.A. Ahmad, S.H. Patel, The impact of tumor location on resection and survival for pancreatic ductal adenocarcinoma. J Surg Res 239, 60–66 (2019)
Q. Ling, X. Xu, P. Ye, H. Xie, F. Gao, Q. Hu, Z. Liu, X. Wei, C. Roder, A. Trauzold, H. Kalthoff, S. Zheng, The prognostic relevance of primary tumor location in patients undergoing resection for pancreatic ductal adenocarcinoma. Oncotarget 8, 15159–15167 (2017)
M.K. Lau, J.A. Davila, Y.H. Shaib, Incidence and survival of pancreatic head and body and tail cancers: A population-based study in the United States. Pancreas 39, 458–462 (2010)
I. Watanabe, S. Sasaki, M. Konishi, T. Nakagohri, K. Inoue, T. Oda, T. Kinoshita, Onset symptoms and tumor locations as prognostic factors of pancreatic cancer. Pancreas 28, 160–165 (2004)
Funding
This study was supported by grants from the National Natural Science Foundation of China (No. 81772639, No. 81802475, No. 81972258, No. 81974376), the Natural Science Foundation of Beijing (No. 7192157), the CAMS Innovation Fund for Medical Sciences (CIFMS) (No.2016-I2M-1-001) and the Non-profit Central Research Institute Fund of the Chinese Academy of Medical Sciences (No. 2018PT32014).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the writing and editing of this manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This research was approved by the Research Medical Ethics Committee of Peking Union Medical College Hospital. Informed consent was obtained from each patient.
Consent for publication
All authors agreed to publish the article.
Competing interests
The authors declare no potential conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, G., Wang, Y., Xiao, J. et al. CREPT serves as a biomarker of poor survival in pancreatic ductal adenocarcinoma. Cell Oncol. 44, 345–355 (2021). https://doi.org/10.1007/s13402-020-00569-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-020-00569-7