Abstract
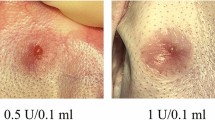
Purpose This study aims to explore the effects of BTXA on the proteins that affect the contraction of fibroblasts. Moreover, the optimal dose of BTXA on hypertrophic scars has not yet been determined. The current study investigated the effect of different doses of BTXA on scar formation. Materials and Methods 18 rabbits’ ears of New Zealand rabbits were used to establish hypertrophic scar model. These wounds were divided into four groups as group B (treated with deferent dosage of BTXA), group T (treated with TAC), group S (not treated), and group C (normal skin). Fibroblast apoptosis was detected by the method of TUNEL. Collagen I, III, and TGF-β1 expression were examined by western blotting method. The immunohistochemical staining and computerized image analysis were used to measure the optical density (OD value) of positive staining of α-SMA and myosin II. Results With increasing BTXA dose, the apoptotic rates increased. Western blot showed that Collagen fibrils and TGF-β1 proteins were expressed lower with the increasing of dose of BTXA (P < 0.05). α-SMA and myosin II in Group B were significantly decreased than those in Group T (P < 0.05), moreover, more myosin II can be reduced with high concentrations of BTXA (2.0 IU). Conclusions It was shown that BTXA could induce fibroblast apoptosis and inhibit the expression of α-SMA and myosin II, which can inhibit FB contraction. It plays a positive role in the treatment of scar.
Similar content being viewed by others
References
Gassner, H. G., D. A. Sherris, and C. C. Otley (2000) Treatment of facial wounds with botulinum toxin a improves cosmetic outcome in primates. Plast. Reconstr. Surg. 105: 1948–1953.
Liu, Y., B. Deng, Y. Zhao, S. Xie, and R. Nie (2013) Differentiated markers in undifferentiated cells: expression of smooth muscle contractile proteins in multipotent bone marrow mesenchymal stem cells. Dev. Growth. Differ. 55: 591–605.
Hallett, M. (1999) One man’s poison—clinical applications of botulinum toxin. N. Engl. J. Med. 341: 118–120.
Jun, J. Y., J. H. Park, C. S. Youn, and J. H. Lee (2018) Intradermal injection of botulinum toxin: a safer treatment modality for forehead wrinkles. Ann. Dermatol. 30: 458–461.
Polak, F., R. Morton, C. Ward, W. A. Wallace, L. Doderlein, and A. Siebel (2002) Double-blind comparison study of two doses of botulinum toxin A injected into calf muscles in children with hemiplegic cerebral palsy. Dev. Med. Child. Neurol. 44: 551–555.
Sherris, D. A. and H. G. Gassner (2002) Botulinum toxin to minimize facial scarring. Facial. Plast. Surg. 18: 35–39.
Tracy, L. E., R. A. Minasian, and E. J. Caterson (2016) Extracellular matrix and dermal fibroblast function in the healing wound. Adv. Wound. Care (New Rochelle). 5: 119–136.
Chen, M., T. Yan, K. Ma, L. Lai, C. Liu, L. Liang, and X. Fu (2016) Botulinum toxin type A inhibits alpha-smooth muscle actin and myosin II expression in fibroblasts derived from scar contracture. Ann. Plast. Surg. 77: e46–49.
Liu, D. Q., X. J. Li, and Weng (2017) Effect of BTXA on inhibiting hypertrophic scar formation in a rabbit ear model. Aesthetic. Plast. Surg. 41: 721–728.
Wang, H., Z. Chen, X. J. Li, L. Ma, and Y. L. Tang (2015) Anti-inflammatory cytokine TSG-6 inhibits hypertrophic scar formation in a rabbit ear model. Eur. J. Pharmacol. 751: 42–49.
Phadke, C. P., A. Y. On, Y. Kirazli, F. Ismail, and C. Boulias (2013) Intrafusal effects of botulinum toxin injections for spasticity: revisiting a previous paper. Neurosci. Lett. 541: 20–23.
Lee, B. J., J. H. Jeong, S. G. Wang, J. C. Lee, E. K. Goh, and H. W. Kim (2009) Effect of botulinum toxin type a on a rat surgical wound model. Clin. Exp. Otorhinolaryngol. 2: 20–27.
Prodromidou, A., M. Frountzas, D. E. G. Vlachos, G. D. Vlachos, I. Bakoyiannis, D. Perrea, and V. Pergialiotis (2015) Botulinum toxin for the prevention and healing of wound scars: A systematic review of the literature. Plast. Surg (Oakv). 23: 260–264.
Gassner, H. G., A. E. Brissett, C. C. Otley, D. K. Boahene, A. J. Boggust, A. L. Weaver, and D. A. Sherris (2006) Botulinum toxin to improve facial wound healing: A prospective, blinded, placebo-controlled study. Mayo. Clin. Proc. 81: 1023–1028.
Xiao, Z. and G. Qu (2012) Effects of botulinum toxin type a on collagen deposition in hypertrophic scars. Molecules. 17: 2169–2177.
Xiao, Z., F. Zhang, W. Lin, M. Zhang, and Y. Liu (2010) Effect of botulinum toxin type A on transforming growth factor beta1 in fibroblasts derived from hypertrophic scar: a preliminary report. Aesthetic. Plast. Surg. 34: 424–427.
Tanaka, J., T. Watanabe, N. Nakamura, and K. Sobue (1993) Morphological and biochemical analyses of contractile proteins (actin, myosin, caldesmon and tropomyosin) in normal and transformed cells. J. Cell. Sci. 104: 595–606.
Bond, J. E., T. Q. Ho, M. A. Selim, C. L. Hunter, E. V. Bowers, and H. Levinson (2011) Temporal spatial expression and function of non-muscle myosin II isoforms IIA and IIB in scar remodeling. Lab. Invest. 91: 499–508.
Vicente-Manzanares, M., X. Ma, R. S. Adelstein, and A. R. Horwitz (2009) Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell. Biol. 10: 778–790.
Acknowledgments
This study was funded by Anhui Science and Technology Research Project (1604a0802078)
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure Statement
The authors declare no conflicts of interest. This study was approved by the Animal Ethics Committee of Anhui Medical University (LLSC20170427) and no informed consent was required for this study.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, J., Liu, D., Li, X. et al. BTXA Could Induce Fibroblast Apoptosis and Inhibit the Expression of α-SMA and Myosin II in Scar Tissue of Rabbit Ears. Biotechnol Bioproc E 25, 699–706 (2020). https://doi.org/10.1007/s12257-019-0431-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-019-0431-9