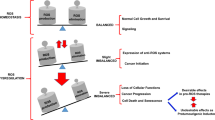
Abstract
Strictly regulated balance between the formation and utilization of reactive oxygen species (ROS) is the basis of normal functioning of organisms. ROS play an important role in the regulation of many metabolic processes; however, excessive content of ROS leads to the development of various disorders, including oncological diseases, as a result of ROS-induced mutations in DNA. In tumors, high levels of oxygen radicals promote cell proliferation and metastasis. On the other hand, high content of ROS can trigger cell death, a phenomenon used in the antitumor therapy. Water- and lipid-soluble antioxidants, as well as antioxidant enzyme systems, can inhibit ROS generation; however, they should be used with caution. Antioxidants can suppress ROS-dependent cell proliferation and metastasis, but at the same time, they may inhibit the death of tumor cells if the antitumor therapeutic agents stimulate oxidative stress. The data on the role of antioxidants in the death of tumor cells and on the effects of antioxidants taken as dietary supplements during antitumor therapy, are contradictory. This review focuses on the mechanisms by which antioxidants can affect tumor and healthy cells.



Similar content being viewed by others
Abbreviations
- GPx:
-
glutathione peroxidase
- HIF:
-
hypoxia-inducible factor
- MAO:
-
monoamine oxidase
- MPT:
-
mitochondrial permeability transition
- ROS:
-
reactive oxygen species
- NF-κB:
-
nuclear factor κB
- NOX:
-
NADPH oxidase
- PCD:
-
programmed cell death
- SOD:
-
superoxide dismutase
- TNF:
-
tumor necrosis factor
References
Hanahan, D., and Weinberg, R. A. (2000) The hallmarks of cancer, Cell, 100, 57-70, doi: https://doi.org/10.1016/s0092-8674(00)81683-9.
Galluzzi, L., Vitale, I., Aaronson, S. A., Abrams, J. M., Adam, D., et al. (2018) Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018, Cell Death Differ., 25, 486-541, doi: https://doi.org/10.1038/s41418-017-0012-4.
Halliwell, B. (1999) Oxygen and nitrogen are pro-carcinogens. Damage to DNA by reactive oxygen, chlorine and nitrogen species: measurement, mechanism and the effects of nutrition, Mutat. Res., 443, 37-52, doi: https://doi.org/10.1016/s1383-5742(99)00009-5.
Andreyev, A. Y., Kushnareva, Y. E., Murphy, A. N., and Starkov, A. A. (2015) Mitochondrial ROS metabolism: 10 years later, Biochemistry (Moscow), 80, 517-531, doi: https://doi.org/10.1134/S0006297915050028.
Gaweska, H., and Fitzpatrick, P. F. (2011) Structures and meanism of the monoamine oxidase family, Biomol. Concepts, 2, 365-377, doi: https://doi.org/10.1515/BMC.2011.030.
Liguori, I., Russo, G., Curcio, F., Bulli, G., Aran, L., et al. (2018) Oxidative stress, aging and diseases, Clin. Interv. Aging, 13, 757-772, doi: https://doi.org/10.2147/CIA.S158513.
Schroder, K., Zhang, M., Benkhoff, S., Mieth, A., Pliquett, R., et al. (2012) Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase, Circ. Res., 110, 1217-1225, doi: https://doi.org/10.1161/CIRCRESAHA.112.267054.
Meng, X. M., Ren, G. L., Gao, L., Yang, Q., Li, H. D., et al. (2018) NADPH oxidase 4 promotes cisplatin-induced acute kidney injury via ROS-mediated programmed cell death and inflammation, Lab. Invest., 98, 63-78, doi: https://doi.org/10.1038/labinvest.2017.120.
Haag-Liautard, C., Coffey, N., Houle, D., Lynch, M., Charlesworth, B., and Keightley, P. D. (2008) Direct estimation of the mitochondrial DNA mutation rate in Drosophila melanogaster, PLoS Biol., 6, e204, doi: https://doi.org/10.1371/journal.pbio.0060204.
Elchuri, S., Oberley, T. D., Qi, W., Eisenstein, R. S., Jackson Roberts, L., et al. (2005) CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life, Oncogene, 24, 367-380, doi: https://doi.org/10.1038/sj.onc.1208207.
Li, Y., Huang, T. T., Carlson, E. J., Melov, S., Ursell, P. C., et al. (1995) Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase, Nat. Genet., 11, 376-381, doi: https://doi.org/10.1038/ng1295-376.
Thannickal, V. J., and Fanburg, B. L. (1995) Activation of an H2O2-generating NADH oxidase in human lung fibroblasts by transforming growth factor beta 1, J. Biol. Chem., 270, 30334-30338, doi: https://doi.org/10.1074/jbc.270.51.30334.
Meier, B., Radeke, H. H., Selle, S., Younes, M., Sies, H., Resch, K., and Habermehl, G. G. (1989) Human fibroblasts release reactive oxygen species in response to interleukin-1 or tumour necrosis factor-alpha, Biochem. J., 263, 539-545, doi: https://doi.org/10.1042/bj2630539.
Redza-Dutordoir, M., and Averill-Bates, D. A. (2016) Activation of apoptosis signalling pathways by reactive oxygen species, Biochim. Biophys. Acta, 1863, 2977-2992, doi: https://doi.org/10.1016/j.bbamcr.2016.09.012.
Kim, S. Y., Park, C., Jang, H. J., Kim, B. O., Bae, H. W., et al. (2019) Antibacterial strategies inspired by the oxidative stress and response networks, J. Microbiol., 57, 203-212, doi: https://doi.org/10.1007/s12275-019-8711-9.
Wu, M. Y., Yiang, G. T., Liao, W. T., Tsai, A. P., Cheng, Y. L., et al. (2018) Current mechanistic concepts in ischemia and reperfusion injury, Cell. Physiol. Biochem., 46, 1650-1667, doi: https://doi.org/10.1159/000489241.
Rodic, S., and Vincent, M. D. (2018) Reactive oxygen species (ROS) are a key determinant of cancer’s metabolic phenotype, Int. J. Cancer, 142, 440-448, doi: https://doi.org/10.1002/ijc.31069.
Irani, K., Xia, Y., Zweier, J. L., Sollott, S. J., Der, C. J., Fearon, E. R., Sundaresan, M., Finkel, T., and Goldschmidt-Clermont, P. J. (1997) Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts, Science, 275, 1649-1652, doi: https://doi.org/10.1126/science.275.5306.1649.
Vafa, O., Wade, M., Kern, S., Beeche, M., Pandita, T. K., Hampton, G. M., and Wahl, G. M. (2002) c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: a mechanism for oncogene-induced genetic instability, Mol. Cell, 9, 1031-1044, doi: https://doi.org/10.1016/s1097-2765(02)00520-8.
Hole, P. S., Pearn, L., Tonks, A. J., James, P. E., Burnett, A. K., Darley, R. L., and Tonks, A. (2010) Ras-induced reactive oxygen species promote growth factor-independent proliferation in human CD34+ hematopoietic progenitor cells, Blood, 115, 1238-1246, doi: https://doi.org/10.1182/blood-2009-06-222869.
Semenza, G. L. (2003) Targeting HIF-1 for cancer therapy, Nat. Rev. Cancer, 3, 721-732, doi: https://doi.org/10.1038/nrc1187.
Chandel, N. S., McClintock, D. S., Feliciano, C. E., Wood, T. M., Melendez, J. A., Rodriguez, A. M., and Schumacker, P. T. (2000) Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing, J. Biol. Chem., 275, 25130-25138, doi: https://doi.org/10.1074/jbc.M001914200.
Aggarwal, V., Tuli, H. S., Varol, A., Thakral, F., Yerer, M. B., et al. (2019) Role of reactive oxygen species in cancer progression: molecular mechanisms and recent advancements, Biomolecules, 9, doi: https://doi.org/10.3390/biom9110735.
Kanbagli, O., Ozdemirler, G., Bulut, T., Yamaner, S., Aykac-Toker, G., and Uysal, M. (2000) Mitochondrial lipid peroxides and antioxidant enzymes in colorectal adenocarcinoma tissues, Jpn. J. Cancer Res., 91, 1258-1263, doi: https://doi.org/10.1111/j.1349-7006.2000.tb00912.x.
Cobbs, C. S., Levi, D. S., Aldape, K., and Israel, M. A. (1996) Manganese superoxide dismutase expression in human central nervous system tumors, Cancer Res., 56, 3192-3195.
Wang, M., Kirk, J. S., Venkataraman, S., Domann, F. E., Zhang, H. J., et al. (2005) Manganese superoxide dismutase suppresses hypoxic induction of hypoxia-inducible factor-1alpha and vascular endothelial growth factor, Oncogene, 24, 8154-8166, doi: https://doi.org/10.1038/sj.onc.1208986.
Nakata, T., Suzuki, K., Fujii, J., Ishikawa, M., Tatsumi, H., et al. (1992) High expression of manganese superoxide dismutase in 7,12-dimethylbenz[a]anthracene-induced ovarian cancer and increased serum levels in the tumor-bearing rats, Carcinogenesis, 13, 1941-1943, doi: https://doi.org/10.1093/carcin/13.10.1941.
Nunes, S. C., and Serpa, J. (2018) Glutathione in varian cancer: a double-edged sword, Int. J. Mol. Sci., 19, doi: https://doi.org/10.3390/ijms19071882.
Chan, J. M., Darke, A. K., Penney, K. L., Tangen, C. M., et al. (2016) Selenium- or vitamin E-related gene variants, interaction with supplementation, and risk of high-grade prostate cancer in select, Cancer Epidemiol. Biomarkers Prev., 25, 1050-1058, doi: https://doi.org/10.1158/1055-9965.EPI-16-0104.
Singh, A., Misra, V., Thimmulappa, R. K., Lee, H., Ames, S., et al. (2006) Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer, PLoS Med., 3, e420, doi: https://doi.org/10.1371/journal.pmed.0030420.
Oberley, L. W. (2001) Anticancer therapy by overexpression of superoxide dismutase, Antioxid. Redox Signal., 3, 461-472, doi: https://doi.org/10.1089/15230860152409095.
Wang, J. Y., Wang, X., Wang, X. J., Zheng, B. Z., Wang, Y., Wang, X., and Liang, B. (2018) Curcumin inhibits the growth via Wnt/beta-catenin pathway in non-small-cell lung cancer cells, Eur. Rev. Med. Pharmacol. Sci., 22, 7492-7499, doi: https://doi.org/10.26355/eurrev_201811_16290.
Isakov, N. (2018) Protein kinase C (PKC) isoforms in cancer, tumor promotion and tumor suppression, Semin. Cancer Biol., 48, 36-52, doi: https://doi.org/10.1016/j.semcancer.2017.04.012.
Knock, G. A., and Ward, J. P. (2011) Redox regulation of protein kinases as a modulator of vascular function, Antioxid. Redox Signal., 15, 1531-1547, doi: https://doi.org/10.1089/ars.2010.3614.
Natarajan, K., Gottipati, K. R., Berhane, K., Samten, B., Pendurthi, U., and Boggaram, V. (2016) Proteases and oxidant stress control organic dust induction of inflammatory gene expression in lung epithelial cells, Respir. Res., 17, 137, doi: https://doi.org/10.1186/s12931-016-0455-z.
Sagun, K. C., Cárcamo, J. M., and Golde, D. W. (2006) Antioxidants prevent oxidative DNA damage and cellular transformation elicited by the over-expression of c-MYC, Mutat. Res., 593, 64-79, doi: https://doi.org/10.1016/j.mrfmmm.2005.06.015.
Gordan, J. D., Thompson, C. B., and Simon, M. C. (2007) HIF and c-Myc: sibling rivals for control of cancer cell metabolism and proliferation, Cancer Cell, 12, 108-113, doi: https://doi.org/10.1016/j.ccr.2007.07.006.
Dolcet, X., Llobet, D., Pallares, J., and Matias-Guiu, X. (2005) NF-κB in development and progression of human cancer, Virchows Arch., 446, 475-482, doi: https://doi.org/10.1007/s00428-005-1264-9.
Johnson, J., Thijssen, B., McDermott, U., Garnett, M., Wessels, L. F., and Bernards, R. (2016) Targeting the RB-E2F pathway in breast cancer, Oncogene, 35, 4829-4835, doi: https://doi.org/10.1038/onc.2016.32.
Nevins, J. R. (2001) The Rb/E2F pathway and cancer, Hum. Mol. Genet., 10, 699-703, doi: https://doi.org/10.1093/hmg/10.7.699.
De Jager, S. M., Maughan, S., Dewitte, W., Scofield, S., and Murray, J. A. (2005) The developmental context of cell-cycle control in plants, Semin. Cell. Dev. Biol., 16, 385-396, doi: https://doi.org/10.1016/j.semcdb.2005.02.004.
Bakshi, S., Bergman, M., Dovrat, S., and Grossman, S. (2004) Unique natural antioxidants (NAOs) and derived purified components inhibit cell cycle progression by downregulation of ppRb and E2F in human PC3 prostate cancer cells, FEBS Lett., 573, 31-37, doi: https://doi.org/10.1016/j.febslet.2004.06.101.
Henry, D., Brumaire, S., and Hu, X. (2019) Involvement of pRb-E2F pathway in green tea extract-induced growth inhibition of human myeloid leukemia cells, Leuk. Res., 77, 34-41, doi: https://doi.org/10.1016/j.leukres.2018.12.014.
Sayin, V. I., Ibrahim, M. X., Larsson, E., Nilsson, J. A., Lindahl, P., and Bergo, M. O. (2014) Antioxidants accelerate lung cancer progression in mice, Sci. Transl. Med., 6, 221ra215, doi: https://doi.org/10.1126/scitranslmed.3007653.
Wiel, C., Le Gal, K., Ibrahim, M. X., Jahangir, C. A., Kashif, M., et al. (2019) BACH1 stabilization by antioxidants stimulates lung cancer metastasis, Cell, 178, 330-345.e322, doi: https://doi.org/10.1016/j.cell.2019.06.005.
Lignitto, L., LeBoeuf, S. E., Homer, H., Jiang, S., Askenazi, M., et al. (2019) Nrf2 activation promotes lung cancer metastasis by inhibiting the degradation of Bach1, Cell, 178, 316-329.e18, doi: https://doi.org/10.1016/j.cell.2019.06.003.
Kumari, S., Badana, A. K., Murali Mohan, G., Shailender, G., and Malla, R. (2018) Reactive oxygen species: a key constituent in cancer survival, Biomark. Insights, 13, 1177271918755391, doi: https://doi.org/10.1177/1177271918755391.
Gogvadze, V., Orrenius, S., and Zhivotovsky, B. (2008) Mitochondria in cancer cells: what is so special about them? Trends Cell Biol., 18, 165-173, doi: https://doi.org/10.1016/j.tcb.2008.01.006.
Eskes, R., Desagher, S., Antonsson, B., and Martinou, J. C. (2000) Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane, Mol. Cell. Biol., 20, 929-935, doi: https://doi.org/10.1128/mcb.20.3.929-935.2000.
Gogvadze, V., Orrenius, S., and Zhivotovsky, B. (2006) Multiple pathways of cytochrome c release from mitochondria in apoptosis, Biochim. Biophys. Acta, 1757, 639-647, doi: https://doi.org/10.1016/j.bbabio.2006.03.016.
Hunter, D. R., and Haworth, R. A. (1979) The Ca2+-induced membrane transition in mitochondria. I. The protective mechanisms, Arch. Biochem. Biophys., 195, 453-459, doi: https://doi.org/10.1016/0003-9861(79)90371-0.
McStay, G. P., Clarke, S. J., and Halestrap, A. P. (2002) Role of critical thiol groups on the matrix surface of the adenine nucleotide translocase in the mechanism of the mitochondrial permeability transition pore, Biochem. J., 367, 541-548, doi: https://doi.org/10.1042/BJ20011672.
Neuzil, J., Wang, X. F., Dong, L. F., Low, P., and Ralph, S. J. (2006) Molecular mechanism of “mitocan”-induced apoptosis in cancer cells epitomizes the multiple roles of reactive oxygen species and Bcl-2 family proteins, FEBS Lett., 580, 5125-5129, doi: https://doi.org/10.1016/j.febslet.2006.05.072.
Ott, M., Robertson, J. D., Gogvadze, V., Zhivotovsky, B., and Orrenius, S. (2002) Cytochrome c release from mitochondria proceeds by a two-step process, Proc. Natl. Acad. Sci. USA, 99, 1259-1263, doi: https://doi.org/10.1073/pnas.241655498.
Kagan, V. E., Bayir, H. A., Belikova, N. A., Kapralov, O., Tyurina, Y. Y., et al. (2009) Cytochrome c/cardiolipin relations in mitochondria: a kiss of death, Free Radic. Biol. Med., 46, 1439-1453, doi: https://doi.org/10.1016/j.freeradbiomed.2009.03.004.
Cai, J., and Jones, D. P. (1999) Mitochondrial redox signaling during apoptosis, J. Bioenerg. Biomembr., 31, 327-334, doi: https://doi.org/10.1023/a:1005423818280.
Cabeca, T. K., de Mello Abreu, A., Andrette, R., de Souza Lino, V., Morale, M. G., et al. (2019) HPV-mediated resistance to TNF and TRAIL is characterized by global alterations in apoptosis regulatory factors, dysregulation of death receptors, and induction of ROS/RNS, Int. J. Mol. Sci., 20, doi: https://doi.org/10.3390/ijms20010198.
Choi, K., Ryu, S. W., Song, S., Choi, H., Kang, S. W., and Choi, C. (2010) Caspase-dependent generation of reactive oxygen species in human astrocytoma cells contributes to resistance to TRAIL-mediated apoptosis, Cell. Death Differ., 17, 833-845, doi: https://doi.org/10.1038/cdd.2009.154.
Giorgio, M., Migliaccio, E., Orsini, F., Paolucci, D., Moroni, M., et al. (2005) Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis, Cell, 122, 221-233, doi: https://doi.org/10.1016/j.cell.2005.05.011.
Hu, W., Zhang, C., Wu, R., Sun, Y., Levine, A., and Feng, Z. (2010) Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function, Proc. Natl. Acad. Sci. USA, 107, 7455-7460, doi: https://doi.org/10.1073/pnas.1001006107.
Sablina, A. A., Budanov, A. V., Ilyinskaya, G. V., Agapova, L. S., Kravchenko, J. E., and Chumakov, P. M. (2005) The antioxidant function of the p53 tumor suppressor, Nat. Med., 11, 1306-1313, doi: https://doi.org/10.1038/nm1320.
Johnson, T. M., Yu, Z. X., Ferrans, V. J., Lowenstein, R. A., and Finkel, T. (1996) Reactive oxygen species are downstream mediators of p53-dependent apoptosis, Proc. Natl. Acad. Sci. USA, 93, 11848-11852, doi: https://doi.org/10.1073/pnas.93.21.11848.
Matoba, S., Kang, J. G., Patino, W. D., Wragg, A., Boehm, M., et al. (2006) p53 regulates mitochondrial respiration, Science, 312, 1650-1653, doi: https://doi.org/10.1126/science.1126863.
Hampton, M. B., and Orrenius, S. (1997) Dual regulation of caspase activity by hydrogen peroxide: implications for apoptosis, FEBS Lett., 414, 552-556, doi: https://doi.org/10.1016/s0014-5793(97)01068-5.
Akram, S., Teong, H. F., Fliegel, L., Pervaiz, S., and Clement, M. V. (2006) Reactive oxygen species-mediated regulation of the Na+-H+ exchanger 1 gene expression connects intracellular redox status with cells’ sensitivity to death triggers, Cell Death Differ., 13, 628-641, doi: https://doi.org/10.1038/sj.cdd.4401775.
Pohl, S. O., Agostino, M., Dharmarajan, A., and Pervaiz, S. (2018) Cross talk between cellular redox state and the antiapoptotic protein Bcl-2, Antioxid. Redox Signal., 29, 1215-1236, doi: https://doi.org/10.1089/ars.2017.7414.
Stockwell, B. R., Friedmann Angeli, J. P., Bayir, H., Bush, A. I., Conrad, M., et al. (2017) Ferroptosis: a regulated cell death nexus linking metabolism, redox biology and disease, Cell, 171, 273-285, doi: https://doi.org/10.1016/j.cell.2017.09.021.
Kinowaki, Y., Kurata, M., Ishibashi, S., Ikeda, M., Tatsuzawa, A., et al. (2018) Glutathione peroxidase 4 overexpression inhibits ROS-induced cell death in diffuse large B-cell lymphoma, Lab. Invest., 98, 609-619, doi: https://doi.org/10.1038/s41374-017-0008-1.
Lubos, E., Loscalzo, J., and Handy, D. E. (2011) Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities, Antioxid. Redox Signal., 15, 1957-1997, doi: https://doi.org/10.1089/ars.2010.3586.
Canli, O., Alankus, Y. B., Grootjans, S., Vegi, N., Hultner, L., et al. (2016) Glutathione peroxidase 4 prevents necroptosis in mouse erythroid precursors, Blood, 127, 139-148, doi: https://doi.org/10.1182/blood-2015-06-654194.
Li, L., Tan, J., Miao, Y., Lei, P., and Zhang, Q. (2015) ROS and autophagy: interactions and molecular regulatory mechanisms, Cell Mol. Neurobiol., 35, 615-621, doi: https://doi.org/10.1007/s10571-015-0166-x.
Abdrakhmanov, A., Kulikov, A. V., Luchkina, E. A., Zhivotovsky, B., and Gogvadze, V. (2019) Involvement of mitophagy in cisplatin-induced cell death regulation, Biol. Chem., 400, 161-170, doi: https://doi.org/10.1515/hsz-2018-0210.
Raza, M. H., Siraj, S., Arshad, A., Waheed, U., Aldakheel, F., Alduraywish, S., and Arshad, M. (2017) ROS-modulated therapeutic approaches in cancer treatment, J. Cancer Res. Clin. Oncol., 143, 1789-1809, doi: https://doi.org/10.1007/s00432-017-2464-9.
Cho, H. D., Lee, J. H., Moon, K. D., Park, K. H., Lee, M. K., and Seo, K. I. (2018) Auriculasin-induced ROS causes prostate cancer cell death via induction of apoptosis, Food Chem. Toxicol., 111, 660-669, doi: https://doi.org/10.1016/j.fct.2017.12.007.
Wu, Q., Deng, J., Fan, D., Duan, Z., Zhu, C., Fu, R., and Wang, S. (2018) Ginsenoside Rh4 induces apoptosis and autophagic cell death through activation of the ROS/JNK/p53 pathway in colorectal cancer cells, Biochem. Pharmacol., 148, 64-74, doi: https://doi.org/10.1016/j.bcp.2017.12.004.
Nicco, C., and Batteux, F. (2017) ROS modulator molecules with therapeutic potential in cancers treatments, Molecules, 23, doi: https://doi.org/10.3390/molecules23010084.
Biasutto, L., Dong, L. F., Zoratti, M., and Neuzil, J. (2010) Mitochondrially targeted anti-cancer agents, Mitochondrion, 10, 670-681, doi: https://doi.org/10.1016/j.mito.2010.06.004.
Kulikov, A. V., Vdovin, A. S., Zhivotovsky, B., and Gogvadze, V. (2014) Targeting mitochondria by alpha-tocopheryl succinate overcomes hypoxia-mediated tumor cell resistance to treatment, Cell. Mol. Life Sci., 71, 2325-2333, doi: https://doi.org/10.1007/s00018-013-1489-8.
Kruspig, B., Nilchian, A., Bejarano, I., Orrenius, S., Zhivotovsky, B., and Gogvadze, V. (2012) Targeting mitochondria by alpha-tocopheryl succinate kills neuroblastoma cells irrespective of MycN oncogene expression, Cell. Mol. Life Sci., 69, 2091-2099, doi: https://doi.org/10.1007/s00018-012-0918-4.
Ito, H., and Matsui, H. (2016) Mitochondrial reactive oxygen species and photodynamic therapy, Laser Ther., 25, 193-199, doi: https://doi.org/10.5978/islsm.16-OR-15.
Ubezio, P., and Civoli, F. (1994) Flow cytometric detection of hydrogen peroxide production induced by doxorubicin in cancer cells, Free Radic. Biol. Med., 16, 509-516, doi: https://doi.org/10.1016/0891-5849(94)90129-5.
Lawenda, B. D., Kelly, K. M., Ladas, E. J., Sagar, S. M., Vickers, A., and Blumberg, J. B. (2008) Should supplemental antioxidant administration be avoided during chemotherapy and radiation therapy? J. Natl. Cancer Inst., 100, 773-783, doi: https://doi.org/10.1093/jnci/djn148.
Isuzugawa, K., Inoue, M., and Ogihara, Y. (2001) Ca2+-dependent caspase activation by gallic acid derivatives, Biol. Pharm. Bull., 24, 844-847, doi: https://doi.org/10.1248/bpb.24.844.
Motterlini, R., Foresti, R., Bassi, R., and Green, C. J. (2000) Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress, Free Radic. Biol. Med., 28, 1303-1312, doi: https://doi.org/10.1016/s0891-5849(00)00294-x.
Moghtaderi, H., Sepehri, H., Delphi, L., and Attari, F. (2018) Gallic acid and curcumin induce cytotoxicity and apoptosis in human breast cancer cell MDA-MB-231, Bioimpacts, 8, 185-194, doi: https://doi.org/10.15171/bi.2018.21.
Kuo, M. L., Huang, T. S., and Lin, J. K. (1996) Curcumin, an antioxidant and anti-tumor promoter, induces apoptosis in human leukemia cells, Biochim. Biophys. Acta, 1317, 95-100, doi: https://doi.org/10.1016/s0925-4439(96)00032-4.
Sant, D. W., Mustafi, S., Gustafson, C. B., Chen, J., Slingerland, J. M., and Wang, G. (2018) Vitamin C promotes apoptosis in breast cancer cells by increasing TRAIL expression, Sci. Rep., 8, 5306, doi: https://doi.org/10.1038/s41598-018-23714-7.
Valter, K., Chen, L., Kruspig, B., Maximchik, P., Cui, H., Zhivotovsky, B., and Gogvadze, V. (2017) Contrasting effects of glutamine deprivation on apoptosis induced by conventionally used anticancer drugs, Biochim. Biophys. Acta Mol. Cell. Res., 1864, 498-506, doi: https://doi.org/10.1016/j.bbamcr.2016.12.016.
Renu, K., Abilash, V. G., Tirupathi Pichiah, P. B., and Arunachalam, S. (2018) Molecular mechanism of doxorubicin-induced cardiomyopathy – an update, Eur. J. Pharmacol., 818, 241-253, doi: https://doi.org/10.1016/j.ejphar.2017.10.043.
Wang, S., Konorev, E. A., Kotamraju, S., Joseph, J., Kalivendi, S., and Kalyanaraman, B. (2004) Doxorubicin induces apoptosis in normal and tumor cells via distinctly different mechanisms. intermediacy of H(2)O(2)- and p53-dependent pathways, J. Biol. Chem., 279, 25535-25543, doi: https://doi.org/10.1074/jbc.M400944200.
Vijay, K., Sowmya, P. R., Arathi, B. P., Shilpa, S., Shwetha, H. J., Raju, M., Baskaran, V., and Lakshminarayana, R. (2018) Low-dose doxorubicin with carotenoids selectively alters redox status and upregulates oxidative stress-mediated apoptosis in breast cancer cells, Food Chem. Toxicol., 118, 675-690, doi: https://doi.org/10.1016/j.fct.2018.06.027.
D’Andrea, G. M. (2005) Use of antioxidants during chemotherapy and radiotherapy should be avoided, CA Cancer J. Clin., 55, 319-321, doi: https://doi.org/10.3322/canjclin.55.5.319.
Sakamoto, K., and Sakka, M. (1973) Reduced effect of irradiation on normal and malignant cells irradiated in vivo in mice pretreated with vitamin E, Br. J. Radiol., 46, 538-540, doi: https://doi.org/10.1259/0007-1285-46-547-538.
Witenberg, B., Kletter, Y., Kalir, H. H., Raviv, Z., Fenig, E., et al. (1999) Ascorbic acid inhibits apoptosis induced by X irradiation in HL60 myeloid leukemia cells, Radiat. Res., 152, 468-478.
Jung, A. Y., Cai, X., Thoene, K., Obi, N., Jaskulski, S., et al. (2019) Antioxidant supplementation and breast cancer prognosis in postmenopausal women undergoing chemotherapy and radiation therapy, Am. J. Clin. Nutr., 109, 69-78, doi: https://doi.org/10.1093/ajcn/nqy223.
Prasad, K. N., Kumar, B., Yan, X. D., Hanson, A. J., and Cole, W. C. (2003) Alpha-tocopheryl succinate, the most effective form of vitamin E for adjuvant cancer treatment: a review, J. Am. Coll. Nutr., 22, 108-117, doi: https://doi.org/10.1080/07315724.2003.10719283.
Alexander, M. S., Wilkes, J. G., Schroeder, S. R., Buettner, G. R., Wagner, B. A., et al. (2018) Pharmacologic ascorbate reduces radiation-induced normal tissue toxicity and enhances tumor radiosensitization in pancreatic cancer, Cancer Res., 78, 6838-6851, doi: https://doi.org/10.1158/0008-5472.CAN-18-1680.
Kennedy, M., Bruninga, K., Mutlu, E. A., Losurdo, J., Choudhary, S., and Keshavarzian, A. (2001) Successful and sustained treatment of chronic radiation proctitis with antioxidant vitamins E and C, Am. J. Gastroenterol., 96, 1080-1084, doi: https://doi.org/10.1111/j.1572-0241.2001.03742.x.
Moss, R. W. (2007) Do antioxidants interfere with radiation therapy for cancer? Integr. Cancer Ther., 6, 281-292, doi: https://doi.org/10.1177/1534735407305655.
Delanian, S., Balla-Mekias, S., and Lefaix, J. L. (1999) Striking regression of chronic radiotherapy damage in a clinical trial of combined pentoxifylline and tocopherol, J. Clin. Oncol., 17, 3283-3290, doi: https://doi.org/10.1200/JCO.1999.17.10.3283.
Jaakkola, K., Lahteenmaki, P., Laakso, J., Harju, E., Tykka, H., and Mahlberg, K. (1992) Treatment with antioxidant and other nutrients in combination with chemotherapy and irradiation in patients with small-cell lung cancer, Anticancer Res., 12, 599-606.
Umegaki, K., Aoki, S., and Esashi, T. (1995) Whole body X-ray irradiation to mice decreases ascorbic acid concentration in bone marrow: comparison between ascorbic acid and vitamin E, Free Radic. Biol. Med., 19, 493-497, doi: https://doi.org/10.1016/0891-5849(95)00033-t.
Lenton, K. J., and Greenstock, C. L. (1999) Ability of human plasma to protect against ionising radiation is inversely correlated with age, Mech. Ageing Dev., 107, 15-20, doi: https://doi.org/10.1016/s0047-6374(98)00128-6.
Acknowledgements
The authors express their gratitude to Prof. B. D. Zhivotovsky for help in working on the manuscript, valuable comments, and suggestions. We apologize to those authors whose publications could not be cited due to the limited size of our review.
Funding
This work was supported by the Russian Science Foundation (project no. 19-14-00122), Russian Foundation for Basic Research (project no. 20-015-00105), Swedish Cancer Foundation (project no. 190345), and Stockholm Cancer Foundation (project no. 181301).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest. This article does not contain description of studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Vostrikova, S.M., Grinev, A.B. & Gogvadze, V.G. Reactive Oxygen Species and Antioxidants in Carcinogenesis and Tumor Therapy. Biochemistry Moscow 85, 1254–1266 (2020). https://doi.org/10.1134/S0006297920100132
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297920100132