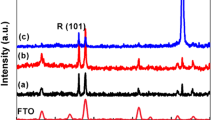
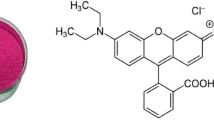
Abstract
3D TiO2 hierarchical nanoflower was synthesized by template-free solvothermal alcoholysis of TiCl3 with the variation of reaction temperatures (130–200 °C). XRD, SEM, BET surface analysis, UV–VIS DR spectroscopy, FTIR analysis were performed to characterize the prepared TiO2 samples. Photocatalytic degradation of model organic pollutant such as methylene blue (MB) dye was investigated using all prepared samples under UV light illumination. Results show that reaction temperature directly affects the anatase phase content of TiO2 samples, crystal structures, crystalline size, particle size, surface area, pore structure, UV absorption capacity, and band gap of the synthesized samples. The sample prepared at reaction temperature 150 °C seems to be most efficient photocatalyst for degradation of MB with rate constant 0.0287 min−1 [~ 20.5 times higher than the sample prepared at 130 °C (0.0014 min−1) and 6.83 times higher than the sample prepared at 200 °C, (0.0042 min−1)]. Optimization of reaction temperature at 150 °C was performed by testing different properties of the synthesized TiO2 nanoflower such as its surface area, organized morphology, bimodal pore-size distribution, and porosity which were synthesized at different solvothermal temperatures (130–200 °C).












Similar content being viewed by others
References
Literathy, P.: Institute for Water Pollution Control. In: Rijtema, E.P., Eliáš, V. (eds.) Regional Approaches to Water Pollution in the Environment, pp. 21–22. Springer, Cham (1996). https://doi.org/10.1007/978-94-009-0345-6
Parris, K.: Impact of agriculture on water pollution in OECD countries: recent trends and future prospects. Int. J. Water Resour. Dev. 27, 33–52 (2011). https://doi.org/10.1080/07900627.2010.531898
Lim, T.T.; Goei, R.: Combined photocatalysis separation processes for water treatment using hybrid photocatalytic membrane reactors. In: Dionysiou, D.D.; Li Puma, G.; Ye, J.; Schneider, J.; Bahnemann, D. (eds.) Photocatalysis: Applications, pp. 130–156. The Royal Society of Chemistry, Cambridge (2016). https://doi.org/10.1039/9781782627104
Clara, M.; Strenn, B.; Ausserleitner, M.; Kreuzinger, N.: Comparison of the behaviour of selected micropollutants in a membrane bioreactor and a conventional wastewater treatment plant. Water Sci. Technol. 50, 29–36 (2004). https://doi.org/10.2166/wst.2004.0305
Gaya, I.U.: Heterogeneous Photocatalysis Using Inorganic Semiconductor Solids. Springer, Dordrecht (2014). https://doi.org/10.1007/978-94-007-7775-0
Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W.: Environmental applications of semiconductor photocatalysis. Chem. Rev. 95, 69–96 (1995). https://doi.org/10.1021/cr00033a004
Hanaor, D.A.H.; Sorrell, C.C.: Review of the anatase to rutile phase transformation. J. Mater. Sci. 46, 855–874 (2011). https://doi.org/10.1007/s10853-010-5113-0
Herrmann, J.M.: Heterogeneous photocatalysis: fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 53(1), 115–129 (1999). https://doi.org/10.1016/S0920-5861(99)00107-8
Zhang, H.; Zhang, H.; Zhu, P.; Huang, F.: Morphological effect in photocatalytic degradation of direct blue over mesoporous TiO2 catalysts. ChemistrySelect 2, 3282–3288 (2017). https://doi.org/10.1002/slct.201601346
Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C.; Di, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C.: Visible-light activation of TiO2 photocatalysts: advances in theory and experiments. J. Photochem. Photobiol. C: Photochem. Rev. 25, 11–29 (2015). https://doi.org/10.1016/j.jphotochemrev.2015.08.003
Andronic, L.; Andrasi, D.; Enesca, A.; Visa, M.; Duta, A.: The influence of titanium dioxide phase composition on dyes photocatalysis. J. Sol-Gel. Sci. Technol. 58, 201–208 (2011). https://doi.org/10.1007/s10971-010-2378-3
Park, H.; Park, Y.; Kim, W.; Choi, W.: Surface modification of TiO2 photocatalyst for environmental applications. J. Photochem. Photobiol. C: Photochem. Rev. 15, 1–20 (2013). https://doi.org/10.1016/j.jphotochemrev.2012.10.001
Sajan, C.P.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J.; Cao, S.: TiO2 nanosheets with exposed 001 facets for photocatalytic applications. Nano Res. 9, 3–27 (2016). https://doi.org/10.1007/s12274-015-0919-3
Wang, W.; Wang, Z.; Liu, J.; Luo, Z.; Suib, S.L.; He, P.; Ding, G.; Zhang, Z.; Sun, L.: Single-step one-pot synthesis of TiO2 nanosheets doped with sulfur on reduced graphene oxide with enhanced photocatalytic activity. Sci. Rep. 7, 46610 (2017). https://doi.org/10.1038/srep46610
Du, J.; Chen, W.; Zhang, C.; Liu, Y.; Zhao, C.; Dai, Y.: Hydrothermal synthesis of porous TiO2 microspheres and their photocatalytic degradation of gaseous benzene. Chem. Eng. J. 170, 53–58 (2011). https://doi.org/10.1016/j.cej.2011.03.027
Ma, L.; Wang, G.; Jiang, C.; Bao, H.; Xu, Q.: Synthesis of core-shell TiO2@g-C3N4 hollow microspheres for efficient photocatalytic degradation of rhodamine B under visible light. Appl. Surface Sci. 430, 263–272 (2018). https://doi.org/10.1016/j.apsusc.2017.07.282
Pan, X.; Zhao, Y.; Liu, S.; Korzeniewski, C.L.; Wang, S.; Fan, Z.: Comparing graphene-TiO2 nanowire and graphene-TiO2 nanoparticle composite photocatalysts. ACS Appl. Mater. Interfaces. 4, 3944–3950 (2012). https://doi.org/10.1021/am300772t
Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K.: Formation of titanium oxide nanotube. Langmuir 14, 3160–3163 (1998). https://doi.org/10.1021/la9713816
Miao, Z.; Xu, D.; Ouyang, J.; Guo, G.; Zhao, X.; Tang, Y.: Electrochemically induced sol-gel preparation of single-crystalline TiO2 nanowires. Nano Lett. 2, 717–720 (2002). https://doi.org/10.1021/nl025541w
Antonelli, D.M.; Ying, J.Y.: Synthesis of hexagonally packed mesoporous TiO2 by a modified sol-gel method. Angew. Chem. Int. Ed. Engl. 34, 2014–2017 (1995). https://doi.org/10.1002/anie.199520141
Ramakrishnan, V.M.; Natarajan, M.; Santhanam, A.; Asokan, V.; Velauthapillai, D.: Size controlled synthesis of TiO2 nanoparticles by modified solvothermal method towards effective photo catalytic and photovoltaic applications. Mater. Res. Bull. 97, 351–360 (2018). https://doi.org/10.1016/j.materresbull.2017.09.017
Yang, H.G.; Liu, G.; Qiao, S.Z.; Sun, C.H.; Jin, Y.G.; Smith, S.C.; Zou, J.; Cheng, H.M.; Lu, G.Q.: Solvothermal synthesis and photoreactivity of anatase TiO2 nanosheets with dominant 001 facets. J. Am. Chem. Soc. 131, 4078–4083 (2009). https://doi.org/10.1021/ja808790p
Endrödi, B.; Kecsenovity, E.; Rajeshwar, K.; Janáky, C.: One-step electrodeposition of nanocrystalline TiO2 films with enhanced photoelectrochemical performance and charge storage. ACS Appl. Energy Mater. 1, 851–858 (2018). https://doi.org/10.1021/acsaem.7b00289
Cheng, H.; Wang, J.; Zhao, Y.; Han, X.: Effect of phase composition{,} morphology{,} and specific surface area on the photocatalytic activity of TiO2 nanomaterials. RSC Adv. 4, 47031–47038 (2014). https://doi.org/10.1039/C4RA05509H
Li, D.; Song, H.; Meng, X.; Shen, T.; Sun, J.; Han, W.; Wang, X.: Effects of particle size on the structure and photocatalytic performance by alkali-treated TiO2. Nanomaterials 10, 1–14 (2020). https://doi.org/10.3390/nano10030546
de Luna, M.D.G.; Garcia-Segura, S.; Mercado, C.H.; Lin, Y.-T.; Lu, M.-C.: Doping TiO2 with CuSO4 enhances visible light photocatalytic activity for organic pollutant degradation. Environ. Sci. Pollut. Res. 27, 24604–24613 (2020). https://doi.org/10.1007/s11356-019-05789-5
Tian, G.; Chen, Y.; Zhou, W.; Pan, K.; Tian, C.; Huang, X.R.; Fu, H.: 3D hierarchical flower-like TiO2 nanostructure: morphology control and its photocatalytic property. CrystEngComm 13, 2994–3000 (2011). https://doi.org/10.1039/c0ce00851f
Liu, L.; Liu, H.; Zhao, Y.P.; Wang, Y.; Duan, Y.; Gao, G.; Ge, M.; Chen, W.: Directed synthesis of hierarchical nanostructured TiO2 catalysts and their morphology-dependent photocatalysis for phenol degradation. Environ. Sci. Technol. 42, 2342–2348 (2008). https://doi.org/10.1021/es070980o
Wu, L.; Qiu, Y.; Xi, M.; Li, X.; Cen, C.: Fabrication of TiO2 nanotubes-assembled hierarchical microspheres with enhanced photocatalytic degradation activity. New J. Chem. 39, 4766–4773 (2015). https://doi.org/10.1039/C5NJ00373C
Fang, B.; Bonakdarpour, A.; Reilly, K.; Xing, Y.; Taghipour, F.; Wilkinson, D.P.: Large-scale synthesis of TiO2 microspheres with hierarchical nanostructure for highly efficient photodriven reduction of CO2 to CH4. ACS Appl. Mater. Interfaces. 6, 15488–15498 (2014). https://doi.org/10.1021/am504128t
Harris, J.; Silk, R.; Smith, M.; Dong, Y.; Chen, W.-T.; Waterhouse, G.I.N.: Hierarchical TiO2 nanoflower photocatalysts with remarkable activity for aqueous methylene blue photo-oxidation. ACS Omega. (2020). https://doi.org/10.1021/acsomega.0c02142
Di Fonzo, F.; Casari, C.S.; Russo, V.; Brunella, M.F.; Li Bassi, A.; Bottani, C.E.: Hierarchically organized nanostructured TiO2 for photocatalysis applications. Nanotechnology (2009). https://doi.org/10.1088/0957-4484/20/1/015604
Zhu, T.; Li, J.; Wu, Q.: Construction of TiO2 hierarchical nanostructures from nanocrystals and their photocatalytic properties. ACS Appl. Mater. Interfaces. 3, 3448–3453 (2011). https://doi.org/10.1021/am2006838
Bian, Z.; Zhu, J.; Wang, J.; Xiao, S.; Nuckolls, C.; Li, H.: Multitemplates for the hierarchical synthesis of diverse inorganic materials. J. Am. Chem. Soc. 134, 2325–2331 (2012). https://doi.org/10.1021/ja210270m
Bian, Z.; Zhu, J.; Li, H.: Solvothermal alcoholysis synthesis of hierarchical TiO2 with enhanced activity in environmental and energy photocatalysis. J. Photochem. Photobiol., C 28, 72–86 (2016). https://doi.org/10.1016/j.jphotochemrev.2016.06.002
Cassiers, K.; Linssen, T.; Mathieu, M.; Bai, Y.Q.; Zhu, H.Y.; Cool, P.; Vansant, E.F.: Surfactant-directed synthesis of mesoporous titania with nanocrystalline anatase walls and remarkable thermal stability. J. Phys. Chem. B 108, 3713–3721 (2004). https://doi.org/10.1021/jp036830r
Bagheri, S.; Mohd Hir, Z.A.; Yousefi, A.T.; Abdul Hamid, S.B.: Progress on mesoporous titanium dioxide: synthesis, modification and applications. Microporous Mesoporous Mater. 218, 206–222 (2015). https://doi.org/10.1016/j.micromeso.2015.05.028
Yu, J.; Su, Y.; Cheng, B.: Template-free fabrication and enhanced photocatalytic activity of hierarchical macro-/mesoporous titania. Adv. Func. Mater. 17, 1984–1990 (2007). https://doi.org/10.1002/adfm.200600933
Ashton, J.F.: Some aspects of the solution chemistry of titanium (III). Some aspects of the solution chemistry of titanium (III). (1977) https://eprints.utas.edu.au/19394/1/whole_AshtonJohnFrederick1977_thesis.pdf. Accessed 8 Aug 2020
Cassaignon, S.; Koelsch, M.; Jolivet, J.P.: From TiCl3 to TiO2 nanoparticles (anatase, brookite and rutile): thermohydrolysis and oxidation in aqueous medium. J. Phys. Chem. Solids 68, 695–700 (2007). https://doi.org/10.1016/j.jpcs.2007.02.020
Kavan, L.; O’Regan, B.; Kay, A.; Grätzel, M.: Preparation of TiO2 (anatase) films on electrodes by anodic oxidative hydrolysis of TiCl3. J. Electroanal. Chem. 346, 291–307 (1993). https://doi.org/10.1016/0022-0728(93)85020-H
Hosono, E.; Fujihara, S.; Kakiuchi, K.; Imai, H.: Growth of submicrometer-scale rectangular parallelepiped rutile TiO2 films in aqueous TiCl3 solutions under hydrothermal conditions. J. Am. Chem. Soc. 126, 7790–7791 (2004). https://doi.org/10.1021/ja048820p
Tan, B.; Zhang, Y.; Long, M.: Large-scale preparation of nanoporous TiO2 film on titanium substrate with improved photoelectrochemical performance. Nanoscale Res. Lett. 9, 1–6 (2014). https://doi.org/10.1186/1556-276X-9-190
Li, J.-G.: TiO2 Nanocrystals: phase selection and morphology control. Int. J. Mater. Sci. Eng. 1, 5–7 (2013). https://doi.org/10.12720/ijmse.1.1.5-7
Danilchenko, S.N.; Kukharenko, O.G.; Moseke, C.; Protsenko, I.Y.; Sukhodub, L.F.; Sulkio-Cleff, B.: Determination of the bone mineral crystallite size and lattice strain from diffraction line broadening. Cryst. Res. Technol. 37, 1234–1240 (2002). https://doi.org/10.1002/1521-4079(200211)37:11 < 1234::AID-CRAT1234 > 3.0.CO;2-X
Langford, J.I.; Wilson, A.J.C.: Scherrer after sixty years: a survey and some new results in the determination of crystallite size. J. Appl. Crystallogr. 11, 102–113 (1978). https://doi.org/10.1107/S0021889878012844
Zhang, H.; Banfield, J.F.: Understanding polymorphic phase transformation behavior during growth of nanocrystalline aggregates: insights from TiO2. J. Phys. Chem. B. 104, 3481–3487 (2000). https://doi.org/10.1021/jp000499j
Fagerlund, G.: Determination of specific surface by the BET method. Matériaux et Constructions 6, 239–245 (1973). https://doi.org/10.1007/BF02479039
Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M.: Characterization of porous solids and powders: surface area, pore size and density. Springer, Cham (2012)
Klobes, P.; Meyer, K.; Munro, R.: Porosity and specific surface area measurements for solid materials. NIST, U. S. Government Printing Office, Washington (2006). https://nvlpubs.nist.gov/nistpubs/Legacy/SP/nistspecialpublication960-17.pdf
Bardestani, R.; Patience, G.S.; Kaliaguine, S.: Experimental methods in chemical engineering: specific surface area and pore size distribution measurements—BET, BJH, and DFT. Can. J. Chem. Eng. 97, 2781–2791 (2019). https://doi.org/10.1002/cjce.23632
Dutta, P.K.; Ray, A.K.; Sharma, V.K.; Millero, F.J.: Adsorption of arsenate and arsenite on titanium dioxide suspensions. J. Colloid Interface Sci. 278, 270–275 (2004). https://doi.org/10.1016/j.jcis.2004.06.015
Chadwick, M.D.; Goodwin, J.W.; Lawson, E.J.; Mills, P.D.A.; Vincent, B.: Surface charge properties of colloidal titanium dioxide in ethylene glycol and water. Colloids Surf. A 203, 229–236 (2002). https://doi.org/10.1016/S0927-7757(01)01101-3
Kosmulski, M.: A literature survey of the differences between the reported isoelectric points and their discussion. Colloids Surf. A 222, 113–118 (2003). https://doi.org/10.1016/S0927-7757(03)00240-1
Herrmann, J.M.: Fundamentals and misconceptions in photocatalysis. J. Photochem. Photobiol., A 216, 85–93 (2010). https://doi.org/10.1016/j.jphotochem.2010.05.015
Herrmann, J.M.: Photocatalysis fundamentals revisited to avoid several misconceptions. Appl. Catal. B 99, 461–468 (2010). https://doi.org/10.1016/j.apcatb.2010.05.012
Madras, G.; McCoy, B.J.: Temperature effects on the transition from nucleation and growth to Ostwald ripening. Chem. Eng. Sci. 59, 2753–2765 (2004). https://doi.org/10.1016/j.ces.2004.03.022
Zhang, H.; Banfield, J.F.: Thermodynamic analysis of phase stability of nanocrystalline titania. J. Mater. Chem. 8, 2073–2076 (1998). https://doi.org/10.1039/A802619J
Ciavatta, L.; Ferri, D.; Riccio, G.: On the hydrolysis of the titanium(IV) ion in chloride media. Polyhedron 4, 15–22 (1985). https://doi.org/10.1016/S0277-5387(00)84215-1
Aruna, S.T.; Tirosh, S.; Zaban, A.: Nanosize rutile titania particle synthesis via a hydrothermal method without mineralizers. J. Mater. Chem. 10, 2388–2391 (2000). https://doi.org/10.1039/b001718n
Kumar, S.G.; Rao, K.S.R.K.: Polymorphic phase transition among the titania crystal structures using a solution-based approach: from precursor chemistry to nucleation process. Nanoscale. 6, 11574–11632 (2014). https://doi.org/10.1039/c4nr01657b
Zhang, H.; Banfield, J.F.: New kinetic model for the nanocrystalline anatase-to-rutile transformation revealing rate dependence on number of particles. Am. Miner. 84, 528–535 (1999). https://doi.org/10.2138/am-1999-0406
Barnard, A.S.; Curtiss, L.A.: Prediction of TiO2 nanoparticle phase and shape transitions controlled by surface chemistry. Nano Lett. 5, 1261–1266 (2005). https://doi.org/10.1021/nl050355m
Gilbert, B.; Zhang, H.; Huang, F.; Finnegan, M.P.; Waychunas, G.A.; Banfield, J.F.: Special phase transformation and crystal growth pathways observed in nanoparticles. Geochem. Trans. 4, 20–27 (2003). https://doi.org/10.1039/b309073f
Finnegan, M.P.; Zhang, H.; Banfield, J.F.: Phase stability and transformation in titania nanoparticles in aqueous solutions dominated by surface energy. J. Phys. Chem. C 111, 1962–1968 (2007). https://doi.org/10.1021/jp063822c
Livage, J.; Henry, M.; Sanchez, C.: Sol-gel chemistry of transition metal oxides. Prog. Solid State Chem. 18, 259–341 (1988). https://doi.org/10.1016/0079-6786(88)90005-2
Wang, C.C.; Ying, J.Y.: Sol-gel synthesis and hydrothermal processing of anatase and rutile titania nanocrystals. Chem. Mater. 11, 3113–3120 (1999). https://doi.org/10.1021/cm990180f
Jolivet, J.P.; Henry, M.; Livage, J.; Bescher, E.: Metal Oxide Chemistry and Synthesis: From Solution to Solid State. Wiley, Hoboken (2000). ISBN 978-0-471-97056-9
Cheng, H.; Ma, J.; Zhao, Z.; Qi, L.: Hydrothermal preparation of uniform nanosize rutile and anatase particles. Chem. Mater. 7, 663–671 (1995). https://doi.org/10.1021/cm00052a010
Gopal, M.; Moberly Chan, W.J.; De Jonghe, L.C.: Room temperature synthesis of crystalline metal oxides. J. Mater. Sci. 32, 6001–6008 (1997). https://doi.org/10.1023/A:1018671212890
Li, Y.; Liu, J.; Jia, Z.: Morphological control and photodegradation behavior of rutile TiO2 prepared by a low-temperature process. Mater. Lett. 60, 1753–1757 (2006). https://doi.org/10.1016/j.matlet.2005.12.012
Huang, X.; Pan, C.: Large-scale synthesis of single-crystalline rutile TiO2 nanorods via a one-step solution route. J. Cryst. Growth 306, 117–122 (2007). https://doi.org/10.1016/j.jcrysgro.2007.04.018
Byrappa, K.; Adschiri, T.: Hydrothermal technology for nanotechnology. Prog. Cryst. Growth Charact. Mater. 53, 117–166 (2007). https://doi.org/10.1016/j.pcrysgrow.2007.04.001
Antony, J.; Nutting, J.; Baer, D.R.; Meyer, D.; Sharma, A.; Qiang, Y.: Size-dependent specific surface area of nanoporous film assembled by core-shell iron nanoclusters. J. Nanomater. 2006, 1–4 (2006). https://doi.org/10.1155/JNM/2006/54961
Kang, X.; Liu, S.; Dai, Z.; He, Y.; Song, X.; Tan, Z.: Titanium dioxide: from engineering to applications. Catalysts 9, 1–32 (2019). https://doi.org/10.3390/catal9020191
Herbert, D.C.; Jones, R.: Localized states in disordered systems. J. Phys. C: Solid State Phys. 4, 1145–1161 (1971). https://doi.org/10.1088/0022-3719/4/10/023
Wang, W.; Liu, P.; Zhang, M.; Hu, J.; Xing, F.: The pore structure of phosphoaluminate cement. Open J. Compos. Mater. 02, 104–112 (2012). https://doi.org/10.4236/ojcm.2012.23012
Yu, J.; Wang, G.; Cheng, B.; Zhou, M.: Effects of hydrothermal temperature and time on the photocatalytic activity and microstructures of bimodal mesoporous TiO2 powders. Appl. Catal. B: Environ. 69, 171–180 (2007). https://doi.org/10.1016/j.apcatb.2006.06.022
Niu, B.; Wang, X.; Wu, K.; He, X.; Zhang, R.: Mesoporous titanium dioxide: synthesis and applications in photocatalysis, energy and biology. Materials. 11, 1–23 (2018). https://doi.org/10.3390/ma11101910
Escobedo-Morales, A.; Ruiz-López, I.I.; de Ruiz-Peralta, M.L.; Tepech-Carrillo, L.; Sánchez-Cantú, M.; Moreno-Orea, J.E.: Automated method for the determination of the band gap energy of pure and mixed powder samples using diffuse reflectance spectroscopy. Heliyon. 5, 1–19 (2019). https://doi.org/10.1016/j.heliyon.2019.e01505
Kumar, P.M.; Badrinarayanan, S.; Sastry, M.: Nanocrystalline TiO2 studied by optical, FTIR and X-ray photoelectron spectroscopy: correlation to presence of surface states. Thin Solid Films 358, 122–130 (2000). https://doi.org/10.1016/S0040-6090(99)00722-1
Okeke, G.; Hammond, R.B.; Joseph Antony, S.: Influence of size and temperature on the phase stability and thermophysical properties of anatase TiO2 nanoparticles: molecular dynamics simulation. J. Nanopart. Res. 15, 1–9 (2013). https://doi.org/10.1007/s11051-013-1584-7
Linsebigler, A.L.; Lu, G.; Yates, J.T.: Photocatalysis on TiO2 surfaces: principles. Mechanisms Select. Results 95, 735–758 (1995). https://doi.org/10.1021/cr00035a013
Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.: Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B: Environ. 31, 145–157 (2001). https://doi.org/10.1016/S0926-3373(00)00276-9
Gnaser, H.; Savina, M.R.; Calaway, W.F.; Tripa, C.E.; Veryovkin, I.V.; Pellin, M.J.: Photocatalytic degradation of methylene blue on nanocrystalline TiO2: surface mass spectrometry of reaction intermediates. Int. J. Mass Spectrom. 245, 61–67 (2005). https://doi.org/10.1016/j.ijms.2005.07.003
Jia, P.; Tan, H.; Liu, K.; Gao, W.: Synthesis, characterization and photocatalytic property of novel ZnO/bone char composite. Mater. Res. Bull. 102, 45–50 (2018). https://doi.org/10.1016/j.materresbull.2018.02.018
Xiao, Q.; Ouyang, L.: Photocatalytic activity and hydroxyl radical formation of carbon-doped TiO2 nanocrystalline: effect of calcination temperature. Chem. Eng. J. 148, 248–253 (2009). https://doi.org/10.1016/j.cej.2008.08.024
Carp, O.; Huisman, C.L.; Reller, A.: Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 32, 33–177 (2004). https://doi.org/10.1016/j.progsolidstchem.2004.08.001
Barzykin, A.V.; Tachiya, M.: Mechanism of charge recombination in dye-sensitized nanocrystalline semiconductors: random flight model. J. Phys. Chem. B. 106, 4356–4363 (2002). https://doi.org/10.1021/jp012957
Qian, R.; Zong, H.; Schneider, J.; Zhou, G.; Zhao, T.; Li, Y.; Yang, J.; Bahnemann, D.W.; Pan, J.H.: Charge carrier trapping, recombination and transfer during TiO2 photocatalysis: an overview. Catal. Today 335, 78–90 (2019). https://doi.org/10.1016/j.cattod.2018.10.053
Zhang, J.; Nosaka, Y.: Mechanism of the OH radical generation in photocatalysis with TiO2 of different crystalline types. J. Phys. Chem. C 118, 10824–10832 (2014). https://doi.org/10.1021/jp501214m
Kakuma, Y.; Nosaka, A.Y.; Nosaka, Y.: Difference in TiO2 photocatalytic mechanism between rutile and anatase studied by the detection of active oxygen and surface species in water. Phys. Chem. Chem. Phys. 17, 18691–18698 (2015). https://doi.org/10.1039/c5cp02004b
Nosaka, Y.; Nosaka, A.: Understanding hydroxyl radical (·OH) generation processes in photocatalysis. ACS Energy Lett. 1, 356–359 (2016). https://doi.org/10.1021/acsenergylett.6b00174
Acknowledgements
Authors would like to acknowledge NIT Durgapur for financial and other administrative support for this reach activity. The authors would also like to extend their heartiest thanks to the funding agency DST Govt. of India for supporting this research through the sponsored project under BRICS Multilateral Call 2017 (Project Grant No. DST/IMRCD/Pilot Call 2/Enviorganic/2018 (G) dated 28.03.2019).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seal, K., Chaudhuri, H., Basu, S. et al. Study on Effect of the Solvothermal Temperature on Synthesis of 3D Hierarchical TiO2 Nanoflower and Its Application as Photocatalyst in Degradation of Organic Pollutants in Wastewater. Arab J Sci Eng 46, 6315–6331 (2021). https://doi.org/10.1007/s13369-020-04988-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-020-04988-4