Abstract
Amplicon sequence fingerprinting of communities in activated sludge systems have provided data revealing the true level of their microbial biodiversity and led to suggestions of which intrinsic and extrinsic parameters might affect the dynamics of community assemblage. Most studies have been performed in China and Denmark, and comparatively little information is available for plants in other countries. This study looked at how the communities of three plants in Victoria, Australia, treating domestic sewage changed with season. All were designed to remove nitrogen microbiologically. They were all located close together to minimise any influence that climate and demographics might have on their operation, and samples were taken at weekly intervals for 12 months. 16S rRNA amplicon sequencing revealed that each plant community was distinctively different to the others and changed over the 12-month sampling period. Many of the factors suggested in other similar studies to be important in determining community composition in activated sludge systems could not explain the changes noted here. The most likely influential factors were considered to be temperature and influent composition reflecting changes in dietary intake by the populations served by each plant, since in all three, the most noticeable changes corresponded to seasonal shifts.
Key points
• Monitoring microbial communities in 3 wastewater treatment plants removing nitrogen
• Temperature is the most influential factor in dynamic changes in community composition.
Similar content being viewed by others
Introduction
There is no doubt that the application of molecular methods has changed considerably our understanding of the microbiology of wastewater treatment plants (WWTP) (Ju and Zhang 2015b). Plants of different configurations designed to remove nitrogen (N) are in operation around the world, and they have one feature in common. All are designed to ensure the coupling of the aerobic oxidation of ammonia (NH3) by the chemolithoautotrophic ammonia-oxidising bacteria (AOB) to nitrite (NO2) (Stein 2019), and then to nitrate (NO3) by the nitrite oxidising bacteria (NOB) (Daims et al. 2015; Daims et al. 2016), with the subsequent sequential reduction by anaerobic respiration by the chemoorganoheterotrophic denitrifying bacteria to N2 using NO3 as an electron acceptor (Lu et al. 2014; McIlroy and Nielsen 2014). Those AOB and NOB identified as major players in nitrification from earlier culture-dependent methods are now viewed largely as laboratory weeds, and instead, others, including many not readily culturable, have emerged as the dominant populations (Daims et al. 2016; Stein 2019).
Thus, most of the dominant AOB in WWTP communities are previously undescribed and often uncultured populations, and ammonia-oxidising Archaea (AOA) are now known to be abundant in WWTP communities (Zhang et al. 2015; Gao et al. 2014; Sauder et al. 2012). It is now clear that Nitrospira and not Nitrobacter are largely responsible for nitrite oxidation, and in many plants are found with previously unknown Nitrotoga populations (Alawi et al. 2009). These are usually less abundant, except in plants operating at low temperatures (Lucker et al. 2014). Members of the NOB have been shown to be phylogenetically diverse containing members of the Proteobacteria and Chloroflexi (Sorokin et al. 2012). They are also physiologically diverse, being able to behave as mixotrophs, in oxidising formate or H2 as an energy source as well as NO2 (Gruber-Dorninger et al. 2015). The interactions between the AOB and NOB are now known to be mutualistic in nature. Each appears microscopically as tight clusters of cells in close proximity to the other, allowing the AOB to provide the NOB with their energy source of NO2 which is toxic to the AOB, while the NOB in turn supply their partners with NH3 generated enzymatically from cyanate and urea (Gruber-Dorninger et al. 2015), and probably other growth factors (Daims et al. 2016).
The long-held view that complete oxidation of NH3 to NO3 required the cooperative interaction of the AOB/AOA and NOB is no longer sustainable. The discovery that certain Nitrospira populations alone can carry out this complete oxidation, known as the complete ammonia-oxidising organisms (Comammox), has led to a radical rethink of their ecological implications in natural communities where these are present. Their abundance and importance in WWTP systems was questioned by Gonzalez-Martinez et al. (Gonzalez-Martinez et al. 2016), but it seems clear now that they are found widely and often in high abundances (Daims et al. 2016; Fan et al. 2017; Koch et al. 2019; Roots et al. 2019). They too exhibit a high level of taxonomic and physiological diversity (Zheng et al. 2019). Equally impressive is our advances in recognising which of the many bacterial populations capable of denitrification are active in activated sludge communities, and which substrates they use as electron donors (McIlroy et al. 2016). Such information has also changed markedly our understanding of the bacterial ecology of denitrification.
Next-generation DNA 16S rRNA amplicon sequencing protocols (Ju and Zhang 2015a) have provided the opportunity to understand which factors, intrinsic and extrinsic, might be responsible for determining the community composition of activated sludge, and more recently the dynamics of community assemblage patterns across both space and time (Xia et al. 2018). Both extrinsic environmental conditions and intrinsic inter-population interactions are considered to play important roles in determining how and why distinctive communities assemble and change (Xia et al. 2018), although little data are currently available in such studies on the impact of the in situ activity of those populations shown by amplicon sequencing to be highly abundant. As most activated sludge community compositions are highly dynamic in ways not well-understood and are likely to reflect complex interactions between many still poorly understood factors, it is difficult currently to arrive at general ecological principles applicable to all.
Certainly, in many early amplicon sequencing studies, most such analyses were largely descriptive, carried out often on single numbers of biomass samples (e.g. Ye and Zhang 2013), and often without details of plant configurations or operating parameters. Later studies have usually employed frequent sampling regimes over extended periods of time. Variables including seasonal and temperature changes (Cai et al. 2020; Griffin and Wells 2017; Jiang et al. 2018a, b; Ju and Zhang 2015a), influent compositions (Jiang et al. 2018a, b), plant location (Griffin and Wells 2017; Zhao et al. 2014), different plant unit processes (Ye and Zhang 2013), wastewater biodegradability (Zhang et al. 2020) and plant operational parameters (Jo et al. 2016). All influence plant community composition, but for each of these variables, not in all the plants that have been examined for them. Methodological studies have included those addressing the often-ignored importance of plant sampling frequencies and length of sampling on the reliability of subsequent statistical data analyses (Jiang et al. 2018a, b). Most published data have been from plants in China and Denmark, and little information globally has been available. However, the report of Wu et al. (Wu et al. 2019) has attempted to address this, shortcoming, where communities from c.a 1200 samples from 269 plants from 6 continents were analysed.
Therefore, while much data are available globally now on population diversities of communities of plants of different configurations (see above), there are scant similar data from plants in Australia. Thus, in this study, the communities of three WWTPs in Victoria configured for removal of nitrogen microbiologically were monitored on a weekly basis over 12 months using Illumina sequencing of 16S rRNA amplicons. The data here show that communities of each of these plants, all located in close proximity in Victoria, Australia, exposed to similar climate conditions, and treating predominantly similar domestic wastes, were distinctively different to each other. Attempts are made here to look at how changing seasons affected their communities and the dynamic shifts in population abundances in each WWTP, which occurred over the study period.
Materials and methods
Plants and sampling
Three wastewater treatment plants chosen for this study were Plant WWTP-A, which is a sequencing batch reactor (SBR) configured as an intermittently decanted aeration lagoon (IDAL) with most of the effluent irrigated together with some wet weather discharge to a local water course. This study is the first to look at the community composition of a plant of this configuration. Plant WWTP-B and plant WWTP-C are both activated sludge plants configured for nitrogen removal. At Plant B, screened and degritted sewage is discharged directly to secondary treatment. The plant has an anoxic selector, uses surface aerators and has a single circular secondary clarifier for solids separation and biomass recirculation. Effluent from plant B discharges to a local watercourse. Plant C incorporates primary sedimentation and anaerobic digestion of sludge, with the effluent totally reused for irrigation. All plants aim at maximising effluent reuse with minimal discharge to the environment and are located within a 40-km radius of each other in southern Victoria, Australia.
Samples were collected from the aeration tanks of each at weekly intervals for 12 months (April 26, 2017–April 18, 2018) and transported, on ice to La Trobe University where they were processed on the same day. Plant operational data used here were collected at the same time from plant employees, and all time points and methods used in this study were at their discretion.
DNA extraction and 16S rRNA gene sequencing
The DNeasy powersoil kit (Qiagen) was used for whole community gDNA extraction of biomass wastewater samples following the manufacturer’s instructions. Concentrations of extracted gDNA were determined with a Qubit 2.0 and normalised to 5 ng/μl. Amplification of the 16S rRNA gene V1-3 variable regions used a 25-cycle PCR using primers 27F and 519R containing the Illumina forward and reverse overhangs respectively (underlined sequence represents the Illumina overhang) 27F (TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGAGAGTTTGATCMTGGCTCAG) and 519R (GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGWATTACCGCGGCKGCTG). PCR amplification was performed using Platinum Taq Polymerase (Invitrogen). The PCR products were cleaned using AMPure XT beads (Beckman Coulter) in accordance with the Illumina protocol. This step was followed by a second PCR with 8 cycles using 5 μl of the first PCR product amplified with Illumina Nextera XT v2 primers to index each sample according to the instructions outlined by Illumina. Amplicon DNA was cleaned with AMPure beads and measured using a Qubit 3.0. Each DNA library was normalised and pooled in equal quantities and sequenced on an Illumina MiSeq using a 600-cycle kit. Raw sequence data have been deposited under BioProject Accession Number PRJNA598073.
DNA sequence analysis
Quality trimming and paired-end read merging was achieved using dada2 (Callahan et al. 2016), although poor reverse-read quality led to some data being discarded. Thus, only the forward reads were used for further analysis. Unique reads that were observed on fewer than two occasions were discarded. Variants of each unique read become a potential representative sequence for a taxon and are referred to here as amplicon sequence variants (ASV). These were assigned using QIIME2 (Bolyen et al. 2018) and the MiDAS (version 2.1) reference database (McIlroy et al. 2017). Data analyses of amplicon data were performed using R packages Ampvis2 (Andersen et al. 2018) and Hmisc (Andersen et al. 2018), while qgraph (Epskamp et al. 2012) and ggcorplot2 were employed for correlation analyses. Upon ASV identification, the data were represented graphically using the R programming language. The heat maps and box plots were generated with the ampvis package.
To explore possible relationships between the relative abundances of selected ASVs and plant operation variables, ASVs were selected based on their high abundances and/or known metabolic importance. Only limited operational data were available for WWTP-B. These parameters were effluent nitrate and ammonia levels, and mixed liquor pH values. Furthermore, of the 48 samples taken for amplicon sequencing, operational data were available for only 35 weeks of the sampling period. Therefore, correlation analyses could only be conducted on samples taken during this period. The WWTP-C plant operational data were available for all samples. These included those mentioned above together with VSS (Volatile Suspended Solids), VS, suspended solids and settled solids, and were determined by the treatment plant operators, using APHA (2005) standard methods.
Correlation analyses were performed using R Studio packages Hmisc (‘Hmisc: harrell miscellaneous’) for analysis, and ggplot (Wickham and Wickham 2007), for visual representations of correlation coefficients.
Results
General comments
Although the compositions of the plant communities were quite different to each other (Fig. 1), members of the phylum Proteobacteria were the most abundant in all three over the study period, with the Betaproteobacteria being the major subgroup. Most descending orders of the relative abundances of the predominant populations in each changed little over the course of this study, as discussed in more detail later. No Archaeal NH3 oxidisers (AOA) or Anammox bacteria were detected in any of the three plant communities over the study period.
(a) PCoA ordination of 16S rRNA amplicons from all mixed liquor samples taken over 12 months from WWTP-A, WWTP-B and WWTP-C. Those from each have formed their individual clusters suggesting few community similarities exist between the three plants. (b) Boxplot showing the average abundances of the top 25 phyla over 12 months of all three plants. The Proteobacteria are split into class
Plots of the three alpha diversity indices for richness (Amplicon Sequence Variance, ASV), biodiversity (Shannon index) and evenness (Simpson evenness) over the 12-month sampling period are given in Fig. S1. The Simpson values show that samples across all three plants had high biodiversity (> 0.9) throughout the 12-month sampling period, except where this fell to 0.8 and 0.7 in the WWTP-C over August/September. No clear explanation for such a change is apparent. None of the measured plant parameters for the WWTP-B showed any statistically significant correlations with any of these indices. However, with the WWTP-C data, statistically significant negative correlations with the Shannon and ASV indices were seen for mixed liquor suspended solids (MLSS) (− 43/0.0049) and SVI (− 0. 53/0.0003) respectively. No correlations were apparent with the Simpson index. With the WWTP-B, negative correlations were established with pH for only the Simpson and ASV indices (− 0.43/0.009 and 0.36/0.32 respectively).
The WWPT-A and WWPT-B both experienced mild foaming incidents over the majority of the study period as discussed below. In addition, WWTP-B experienced two disruptive events, a plant washout caused by heavy rain and a breakdown in the aeration system for one day each. No samples were collected when these occurred. The WWTP-A did not experience any operational problems. Each plant had a community of bulking and foaming bacteria, which showed little change in relative abundances of the more abundant members over the course of the year (Fig. S2). In all three systems, the Chloroflexi Ca. Villigracilis (Nierychlo et al. 2019) was present at high abundance, being especially predominant in the WWTP-B. Most of the others detected were at lower abundances, except for Kouleothrix in WWTP-A and Gordonia amarae–like organisms in WWTP-B, where they were the most likely cause of the foaming incident seen there (Fig. 2a). Microscopy showed the foam in WWTP-C was caused probably by Microthrix parvicella (Fig. 2b), a microaerophilic filament, preferring conditions of low D.O. (Seviour and Nielsen 2010). However, apart from during August and October, this filament was not among the more abundant filamentous bacteria in the mixed liquor.
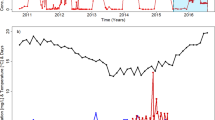
Very little bulking occurred in the WWTP-A, with the only exception being a short period over December and March, although even then, the SVI values never exceeded 180 ml g−1 (Fig. S3). It seems likely that Kouleothrix, a known bulking bacterium (Speirs et al. 2019) was the filament responsible, since it was at high abundances in the period of high SVI values (Fig. 4). On the other hand, bulking sludge was more frequent in the WWTP-B, with mild bulking periods occurring throughout the year, but again SVI readings were never > 200 ml g−1 (Fig. S3). Its filament community was also of low diversity and the most likely bulking candidate was again probably Kouleothrix (Fig. S2). Based on SVI data, the WWTP-C plant had serious bulking problems throughout the study period, where SVI values often reached > 300 ml g−1. Of the three most abundant filaments seen there, the Chloroflexi Villigracilis seems not to be the likely causative filament (Fig. S2), since, as with Ca. Promineofilum, it rarely extends from the floc surface (Nierychlo et al. 2019). Sarcinithrix, known like Kouleothrix to form interfloc bridges of filament bundles (Nierychlo et al. 2019; Speirs et al. 2019), may therefore be involved.
Microbial community composition and dynamics of the WWTP-A
Operational data showed the WWTP-A functioned well over the study period, with high COD and N removal (effluent NH3 always < 2 mg l−1). PCoA ordinations were used to understand how the communities changed on a monthly basis across the year-long study, highlighting their similarities and differences (Fig. 3). Samples taken within the same month generally clustered together, suggesting no major shifts in their community compositions. With samples from different months, there was a distinct shift in cluster arrangements, as seen with those taken from September and October (Fig. 3). The data show that communities examined in November were similar to those for October, while the December communities had clearly changed again, more closely resembling now those observed at the beginning of the study. Toward the end (April 18), the communities appeared to show similarity to those present in 2017. These data suggest that seasonal changes had a marked influence on the community biodiversity of this plant, probably from either change in seasonal temperatures, which ranged from 25 °C in the summer months to 9 °C in the winter, or seasonal dietary changes that contributed to the treatment plants resulting in modifying the influent.
PCoA ordination of the WWTP-A communities at monthly intervals over the twelve-month period. Colours represent the different months and shapes represent different seasons. Similarity between samples is observed by the distance between dots: the greater the distance the greater the dissimilarity. The shapes represent the shading covered by the outermost samples for each month
The heat map data (Fig. 4) show that most populations detected were at low abundances, which varied little over the study period. Among the more predominant genera was Kouleothrix sp., a filamentous member of the Chloroflexi, known to cause bulking in activated sludge systems (Speirs et al. 2019; Nittami et al. 2019). A study from Japan (Nittami et al. 2020) suggests that this filament may be favoured at lower temperatures (< 20°). These filaments are characterised by having large numbers of attached epiphytic bacterial particles, which may explain in part the high percentage abundances of members of the Saprospiraceae in this community (Kong et al. 2007; Xia et al. 2008). However, their relative abundances did not always parallel each other, suggesting that other free-living saprobic Saprospiraceae, commonly found in activated sludge, were present (McIlroy and Nielsen 2014). The filamentous Haliscomenobacter hydrossis, a member of this family, was not detected.
However, one high SVI period (January 17, 2018–February 21, 2018) did correspond to substantial increases in abundance of the Chloroflexi B3 65 population over the same period. This filament is reported to be one of the more common Chloroflexi filaments in Danish wastewater treatment plants, although whether it causes bulking is uncertain (S. McIlroy, personal communication).
Although abundances of the Nitrospirae over the course of the study period decreased, they were present consistently in the community. Very low percent relative abundances of the Nitrosomonas AOB bacteria meant they were detected in only a small number of the plant samples (> 0.01% abundance in a total of six samples from 46) (Fig. 5). Equally, the effluent profiles showing low levels of NO3 (Fig. S1) of < 4 mg l−1 suggest denitrification activity in this plant, and several of the denitrifying bacteria commonly found in activated sludge plants (McIlroy et al. 2016) were detected, including both Rhodoferax and Haliangium.
One unexpected finding was the relatively high abundance (1–2% abundance) of members of the genus Ferribacterium, which were also seen less abundantly later in the study period in WWTP-A. This plant is dosed routinely with ferric sulphate (c.a 250 l every month) to lower chemically the levels of phosphorus in the final plant effluent. Both Ferribacterium and Geothrix use ferric iron (Fe(III)) as an electron acceptor in anaerobic respiration. Less surprising was the presence of the polyphosphate-accumulating organism (PAO) Ca. Accumulibacter at relatively high percentage abundances (0.1–0.5% abundance). Even though this plant was not designed to remove phosphate microbiologically, exposure of the biomass to alternating aerobic–anaerobic/anoxic conditions may provide them with a selective advantage (Seviour et al. 2003), where they may also contribute to denitrification (Saad et al. 2016). Of the Propionivibrio species detected in this community (Fig. 4), Candidatus Propionivibrio aalborgensis (Albertsen et al. 2016) may be among them, and it too has the phenotype of a glycogen accumulating organism (GAO) (Nielsen et al. 2019).
Microbial community composition and dynamics of the WWTP-B
As with the WWTP-A, both COD and NH3 removal were high during the study period (Fig. S1), suggesting efficient plant performance. PCoA ordination analyses reveal the trends in monthly changes in community compositions, which underwent slow and gradual shifts during the first 4 months of the study (Fig. 6). However, drastic changes then appeared to occur during August 2017 and September 2017 as summer approached. This shift is most clearly shown by the relative placement of communities from December through to April 2018, where their close clustering indicates a level of community stabilisation. As with the WWTP-A, these shifts in community structure appear to parallel seasonal changes, and so the same factors of changing temperature and possibly influent characteristics may also apply.
The genus-based heat maps show markedly different community compositions to those from the WWTP-A detailed above (Fig. 4). Now, neither Kouleothrix nor Saprospiraceae populations were detected at such high abundances, and the most abundant populations over the year were Dechloromonas population, with especially large increases over the summer period (Fig. 5), where it contributed up to 20% of the entire bacterial community reads. The genus Dechloromonas includes a diverse collection of strains, some known to be denitrifying bacteria in WWTP (McIlroy et al. 2016), while others behave as PAO (Nielsen et al. 2019). However, their contributions to P removal in full-scale WWTP are considered to be low (Nielsen et al. 2019). The reasons for such increases in Dechloromonas abundances are unclear. Several of the more dominant populations, some of which are seen also in the other two plants, are still poorly understood and characterised (e.g. Chloroflexi SM1F-10, Bacteroidetes PHOS HE-28, Bacteroidetes PHOS-HE51 (OTU143)), and so attempting to explain why they occur at such high abundances is problematic. Clearly, as in WWTP-A communities, high relative abundances of the Nitrospirae, again decreasing across the sampling period, and filamentous Chloroflexi including members of Chloroflexi B3-65 (see above) were among the more dominant populations (Fig. 5).
Operational data profiles from the plant suggest high AOB and NOB and denitrifying bacterial activities, with low residual effluent NH3 values (Fig. S1). Unlike the WWTP-A, Nitrosomonas AOB populations were detected in most samples, albeit at lower abundances than the NOB Nitrospira (0.4–0.7% and 0.9–11%, respectively), and alone among the three plants, Nitrotoga NOB were detected at low abundances, but unexpectedly during the warmer months of the year (Fig. 7). As with the WWTP-A, PAO populations were present, but instead of the betaproteobacterial Ca. Accumulibacter phosphatis, the PAOs were Tetrasphaera spp., an Actinobacterium thought to occupy a different niche to Accumulibacter in WWTP (Nguyen et al. 2015). Evidence suggests it too may denitrify.
Bacterial communities in the WWTP-C
The WWTP-C was the largest of the three plants examined in this study and showed the poorest performance for N removal and general operational behaviour. For example, effluent NH3 values frequently exceeded 10 mg−1, and occasionally, they reached > 20 mg−1 while effluent NO3 levels were generally > 15 mg−1 (Fig. S1). It uses tapered aeration, and so DO levels would vary through the plant, being expected to be very low at the head of the plant where raw influent enters the plant. As a consequence, little nitrification activity might be expected there. Treated effluent is used for irrigation purposes, and so such final high effluent NH3 values are of little environmental concern (P. Griffiths, personal communication). Apart from a constant low-level foaming problem, this plant did not experience any major operational perturbations during this study. As with the other two plants, PCoA analyses (Fig. 8) showed how the bacterial communities changed quite markedly during the study period. They reveal a sharp compositional transition from spring (Oct 2017) into the 2017/2018 (December–January, the summer periods). In other words, within the space of a week, the communities changed sufficiently so that individual clusters for each of these months were still visible. At the beginning of 2018, the community structure then appeared to stabilise, as shown by the samples from the following 4 months clustering closely together. Again, these patterns seem to follow changes in the seasons, and so, as with both other plants, may reflect changes in temperature and/or diet.
PCoA ordination of the WWTP-C communities at monthly intervals over the 12-month period. Colours represent the different months and shapes represent different seasons. Similarity between samples is observed by the distance between dots: the greater the distance, the greater the dissimilarity. The shapes represent the shading covered by the outermost samples for each month
In addition, and as seen with the other two plants, the community compositions of the WWTP-C at the genus level were strikingly distinctive, especially in terms of the predominant populations there (Fig. 9). However, Thauera and Simplicispira, probably S. limi, known to occur in wastewater treatment plants (McIlroy et al. 2016; Lu et al. 2014) both of which are denitrifiers, and Dechloromonas, also able to denitrify, were as abundant as they were in WWTP-A and WWTP-B. The most abundant genus in the plant over the 12-month study period was Zoogloea, and although present at lower abundances in the WWTP-B plant (Fig. 5), members of this genus in WWTP-C reached over 60% of the reads of the entire bacterial communities from April 2017 until December 2017, an abundance only rarely been matched in data from other WWTPs. However, these values fell markedly subsequently. Some members of the genus Zoogloea are known denitrifiers and characteristic producers of excessive amounts of exopolysaccharides, especially under growth conditions like high C:N ratios (Subramanian et al. 2010). However, after a primary sedimentation step, measured influent N levels suggest this is not the explanation for such a dominance. Exopolysaccharide production in Zoogloea is known to be favoured by conditions leading to unbalanced growth arising from nutritional stress, and so it is quite possible that other substrate limitations like low reactor pO2 partly arising from the high readily biodegradable substrate levels in the plant may explain their predominance.
Microscopically, the Zoogloea existed as separate individual rods embedded in a slimy matrix of exopolysaccharide, although ‘fingered’ forms were seen too. Their strongly hydrophobic nature means they may cause the problem of ‘non-filamentous sludge bulking’, although there was no evidence that this was happening in WWTP-C. Their importance in floc formation is probably overstated, but they may denitrify. Despite the presence in the community of a diverse collection of denitrifiers, effluent NO3 levels were consistently high, suggesting little denitrification occurs in this plant (Fig. S1). This plant has very small anoxic zone, so only those chemoorganoheterotrophic denitrifying populations able to utilise the readily biodegradable substrates there can participate. Also, because of primary sedimentation, the amount of organic substrates available in the plant may be too low for high denitrification activity.
As with the WWTP-B, many of the more dominant populations in this plant (for example mle1-27) are either poorly understood or uncharacterised, or could only be identified down to family level or less. Hence, their importance remains unclear.
While Nitrospira sublineage II members were highly abundant in all samples examined here, with little changes in their abundance, members of the AOB Nitrosomonas were rarely detected and then always at low abundances (0–0.5% relative abundances) (Fig. 7). Highly abundant Accumulibacter and less abundant Tetrasphaera were both detected, again showing that this plant has a capacity to remove phosphate microbiologically.
As mentioned earlier, and as with WWPT-B, the WWTP-A had frequent foaming incidents. The heat map of foam samples (Fig. S4) shows that the most abundant sequences were from Zoogloea. This organism is likely to end up in any foam because of its hydrophobicity, carrying with it attached biomass, where the large Gram-negative microcolonies shown in Fig. 2 are likely to be of the amorphous form of Zoogloea. Clearly, the Gram-positive Gordonia amarae–like organisms (GALO) (Fig. 2) dominate the foam microbiome, and hence are most probably the foaming bacteria. Acinetobacter spp. also detected here in the amplicon library (S4), but are not distinctively recognisable, and are seen frequently in foam samples especially during plant start-up (Petrovski et al. 2011). The three heat maps illustrating the shifts in bacterial populations of these foams are shown in Fig. S4.
Discussion
The three plants studied here were selected deliberately to avoid as much as possible extrinsic differences between them in terms of their influent feed compositions, regional demographics and weather conditions. It seems likely that the marked differences in community compositions between the three plants must reflect largely the differences in their individual plant configurations and operational parameters. On the other hand, dynamic changes in community composition and individual population abundances within each plant are probably determined more by changes in intrinsic population behaviour, and reflected in how competitive each population is in being able to maintain the highest growth rates under the changing conditions encountered. Such challenges include coping with uncontrollable changes in abiotic parameters like temperature, pH and pO2 and with inevitable periods of starvation by their abilities to synthesise storage products. Their positive or negative interactions with other members of the community will also be influential, and together, these will determine to a large extent their ability to survive.
There has been an increasing number of published studies based on relative population abundances, but not many on their corresponding in situ activities, and how these and the complex dynamic interactions between the individual populations may affect community assemblage (Xia et al. 2018). However, these interactions appear from the literature to be plant-specific, so no clear general models have so far emerged. Furthermore, explanations for the underlying mechanisms involved in community assemblage studies have not been forthcoming, and attempts to associate particular intrinsic and extrinsic dynamics with changes in population abundances in these highly complex systems are often unconvincing.
A more potentially productive way of achieving this may be to use the approach detailed by Singleton et al. (2020), where they have used high-quality metagenome-assembled genome analyses together with techniques to characterise in situ physiology, to connect community structure to population function. They looked at Danish plants, but the techniques can be applied to any plant. Eventually it may become possible to engineer activated sludge microbiomes using the strategies suggested by Lawson et al. (2019).
Zhang et al. (2020) have suggested that the biodegradability of the influent may play a major role in determining community composition and hence plant performance. They argued that communities of plants receiving an influent with either a high or low BOD/COD ratio had lowered microbial diversity and formed large complex networks. A BOD/total COD influent ratio of 0.5 was considered to be optimal. With low BOD/total COD ratios, the influent would contain large amounts of non-biodegradable substrates, ensuring effluents with high COD levels, and little substrate availability for chemoorganoheterotrophic denitrification, as seen possibly with the WWTP-C. The impact of the BOD/COD ratios on activated sludge plant performance has been the subject of other studies (Ju and Zhang 2015a; Xia et al. 2016), but their impact on the dynamics of community composition is poorly understood, and no attempt has been made to determine which COD fraction (e.g. readily biodegradable or slowly biodegradable) is influential. Of the three plants examined here, only such data were available from WWTP-B. They show that on a few occasions, this BOD/total COD ratio was c.a 0.5, but for most of the year it was < 0.4 (Fig. S1). Yet, there appeared to be no striking changes in the community composition in this plant at the lower BOD/total COD ratios, and the effluent COD values were consistently low over the 12-month period (Fig. S1).
With the exception of the WWTP-B plant communities, both WWTP-C and especially WWTP-A communities contained very few 16S rRNA amplicons traceable to Nitrosomonas and other nitroso AOB. Bearing in mind such low abundances of Nitrosomonas sequences in all three systems, the question of which nitroso AOB are present to oxidise NH3 needs to be addressed, an oxidation step clearly taking place as shown by the low plant effluent NH3 and total nitrogen levels, especially in WWTP-A and WWTP-B (Fig. S1). One possible explanation is that the Nitrospira comammox–nitrifying bacteria are responsible for ammonia and at least partial nitrite oxidation. No direct evidence for their presence is presented here, as 16S rRNA amplicon sequencing will not distinguish them from other Nitrospira populations (Pjevac et al. 2017). However, some indirect evidence may support their involvement. Thus, high abundances of Nitrospira sublineage II in WWTP-C may be relevant since the most commonly occurring environmental Comammox are members of this phylogenetic grouping (Daims et al. 2016; Koch et al. 2019; Zhao et al. 2019). It is now generally agreed that the Comammox nitrifiers are advantaged selectively over the other nitrifiers under stressful conditions, especially low DO levels (Camejo et al. 2017; Koch et al. 2019; Roots et al. 2019). All three plants recorded mixed liquor DO > 1mg l −1 (data not presented) although these readings were obtained from a single DO electrode in each case. Consequently, it is likely that the values obtained did not necessarily reflect DO values throughout the plants, and because of the need to reduce operational costs, all three are considered to be under-aerated for complete nitrification (P. Griffiths, personal communication). Presence of M. parvicella and Zoogloea at WWTP-C, both reflections of operating at low DO (see above), may support this proposal. Clearly amo-qPCR-based methods need to be applied to resolve whether Comammox are actively involved in ammonia oxidation here (Beach and Noguera 2019; Wang et al. 2018; Zhao et al. 2019).
The data here serve to reinforce the view that reaching models generally applicable to all plants in attempts to explain which factors play major roles in determining plant community assemblages is a complex challenge. They also suggest that the temporal dynamics of the three plants seem to be influenced to a large extent by temperature and/or qualitative and quantitative changes in the nature of foods consumed by the populations serving each plant, as seasons change. However, to confirm the trends shown here, additional survey data should be collected over an extended period of more than 1 year. Certainly, temperature has been viewed as one of the more important influences in many other studies, including that of Flowers et al. (2013) and especially the global survey data generated by the Global Water Microbiome Consortium (Wu et al. 2019), but how economically feasible it might be to manipulate community composition of full-scale plants by varying the reactor temperature is clearly problematic.
References
Alawi M, Off S, Kaya M, Spieck E (2009) Temperature influences the population structure of nitrite-oxidizing bacteria in activated sludge. Environ Microbiol Rep 1:184–190
Albertsen M, McIlroy SJ, Stokholm-Bjerregaard M, Karst SM, Nielsen PH (2016) “Candidatus Propionivibrio aalborgensis”: a novel glycogen accumulating organism abundant in full-scale enhanced biological phosphorus removal plants. Front Microbiol 7:1033
Andersen KS, Kirkegaard RH, Karst SM, Albertsen M (2018) ampvis2: an R package to analyse and visualise 16S rRNA amplicon data bioRxiv https://doi.org/10.1101/299537
APHA (2005) Standard methods for the examination of water and wastewater (21st Edn) American Public Health Association/American Wastewater Works Association/Water Environment Federation, Washington DC
Beach NK, Noguera DR (2019) Design and assessment of species-level qPCR primers targeting Comammox. Front Microbiol 10:36
Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet C, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F et al (2019) Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nature Biotechnology 37: 852-857
Cai X, Mao Y, Xu J, Tian L, Wang Y, Iqbal W, Yang B, Liu C, Zhao X, Wang Y (2020) Characterizing community dynamics and exploring bacterial assemblages in two activated sludge systems. Appl Microbiol Biotechnol 104:1795–1808
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP (2016) DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583
Camejo PY, Domingo JS, McMahon KD, Noguera DR (2017) Genome-enabled insights into the ecophysiology of the Comammox bacterium “Candidatus Nitrospira nitrosa”. Msystems 2:e00059-17
Daims H, Lebedeva EV, Pjevac P, Han P, Herbold C, Albertsen M, Jehmlich N, Palatinszky M, Vierheilig J, Bulaev A, Kirkegaard RH, von Bergen M, Rattei T, Bendinger B, Nielsen PH, Wagner M (2015) Complete nitrification by Nitrospira bacteria. Nature 528:504–509
Daims H, Lucker S, Wagner M (2016) A new perspective on microbes formerly known as nitrite-oxidizing bacteria. Trends Microbiol 24:699–712
Epskamp S, Cramer AOJ, Waldorp LJ, Schmittmann VD, Borsboom D (2012) qgraph: Network Visualizations of Relationships in Psychometric Data. J Stat Softw 48:1–18
Fan XY, Zhu Y, Gu PF, Li YM, Xiao GQ, Song DX, Wang YW, He R, Zheng HJ, Li Q (2017) Bacterial community compositions of propylene oxide saponification wastewater treatment plants. RSC Adv 7:22347–22352
Flowers JJ, Cadkin TA, McMahon KD (2013) Seasonal bacterial community dynamics in a full-scale enhanced biological phosphorous removal plant. Water Res 47:7019–7031
Gao JF, Luo X, Wu GX, Li T, Peng YZ (2014) Abundance and diversity based on amoA genes of ammonia-oxidizing archaea and bacteria in ten wastewater treatment systems. Appl Microbiol Biotechnol 98:3339–3354
Gonzalez-Martinez A, Rodriguez-Sanchez A, van Loosdrecht MCM, Gonzalez-Lopez J, Vahala R (2016) Detection of comammox bacteria in full-scale wastewater treatment bioreactors using tag-454-pyrosequencing. Environ Sci Pollut Res 23:25501–25511
Griffin JS, Wells GF (2017) Regional synchrony in full-scale activated sludge bioreactors due to deterministic microbial community assembly. ISME J 11:500–511
Gruber-Dorninger C, Pester M, Kitzinger K, Savio DF, Loy A, Rattei T, Wagner M, Daims H (2015) Functionally relevant diversity of closely related Nitrospira in activated sludge. ISME J 9:643–655
Jiang XT, Ye L, Ju F, Li B, Ma LP, Zhang T (2018a) Temporal dynamics of activated sludge bacterial communities in two diversity variant full-scale sewage treatment plants. Appl Microbiol Biotechnol 102:9379–9388
Jiang XT, Ye L, Ju F, Wang YL, Zhang T (2018b) Toward an intensive longitudinal understanding of activated sludge bacterial assembly and dynamics. Environ Sci Technol 52:8224–8232
Jo SJ, Kwon H, Jeong SY, Lee CH, Kim TG (2016) Comparison of microbial communities of activated sludge and membrane biofilm in 10 full-scale membrane bioreactors. Water Res 101:214–225
Ju F, Zhang T (2015a) Experimental design and bioinformatics analysis for the application of metagenomics in environmental sciences and biotechnology. Environ Sci Technol 49:12628–12640
Ju F, Zhang T (2015b) Bacterial assembly and temporal dynamics in activated sludge of a full-scale municipal wastewater treatment plant. ISME J 9:683–695
Koch C, Huber KJ, Bunk B, Overmann J, Harnisch F (2019) Trophic networks improve the performance of microbial anodes treating wastewater. NPJ Biofilms Microbiomes 5:27
Kong Y, Xia Y, Nielsen JL, Nielsen PH (2007) Structure and function of the microbial community in a full-scale enhanced biological phosphorus removal plant. Microbiology 153:4061–4073
Lawson CE, Harcombe WR, Hatzenpichler R, Lindemann SR, Löffer FE, O’Malley MAO, Martin HG, Pfleger BF, Raskin L, Venturelli S, Weissbrodt DG, Noguera DR, McMahon KD (2019) Common principles and best practices for engineering microbiomes. Nat Rev 17:725–741. https://doi.org/10.1038/s41579-019-0255-9
Lu HJ, Chandran K, Stensel D (2014) Microbial ecology of denitrification in biological wastewater treatment. Water Res 64:237–254
Lucker BF, Hall CC, Zegarac R, Kramer DM (2014) The environmental photobioreactor (ePBR): an algal culturing platform for simulating dynamic natural environments. Algal Res Biomass Biofuels Bioprod 6:242–249
McIlroy SJ, Nielsen PH (2014) The family Saprospiraceae. In: The Prokaryotes. Springer Verlag, Berlin, pp 863–889
McIlroy SJ, Starnawska A, Starnawski P, Saunders AM, Nierychlo M, Nielsen PH, Nielsen JL (2016) Identification of active denitrifiers in full-scale nutrient removal wastewater treatment systems. Environ Microbiol 18:50–64
McIlroy SJ, Kirkegaard RH, McIlroy B, Nierychlo M, Kristensen JM, Karst SM, Albertsen M, Nielsen PH (2017) MiDAS 2.0: an ecosystem-specific taxonomy and online database for the organisms of wastewater treatment systems expanded for anaerobic digester groups. Database (Oxford) 2017
Nguyen HTT, Kristiansen R, Vestergaard M, Wimmer R, Nielsen PH (2015) Intracellular accumulation of glycine in polyphosphate-accumulating organisms in activated sludge, a novel storage mechanism under dynamic anaerobic-aerobic conditions. Appl Environ Microbiol 81:4809–4818
Nielsen PH, McIlroy SJ, Albertsen M, Nierychlo M (2019) Re-evaluating the microbiology of the enhanced biological phosphorus removal process. Curr Opin Biotechnol 57:111–118
Nierychlo M, Milobedka A, Petriglieri F, McIlroy B, Nielsen PH, McIlroy SJ (2019) The morphology and metabolic potential of the Chloroflexii in full-scale activated sludge wastewater treatment plants. FEMS Microbiol Ecol 95:fiy228
Nittami T, Shoji T, Koshiba Y, Noguchi M, Oshiki M, Kuroda M, Kindaichi T, Fukuda J, Futoshi K (2019) Investigation of prospective factors that control Kouleothrix (Type 1851) filamentous bacterial abundance and their correlation with sludge settleability in full-scale wastewater treatment plants. Process Saf Environ Prot 124:137–142
Nittami T, Kasakura R, Kobayashi T, Suzuki K, Koshiba Y, Fukuda J, Takeda T, Kurisu F, Rice D, Petrovski S, Seviour R (2020) Exploring the operating factors controlling Kouleothrix (type 1851), the dominant filamentous bacterial population, in a full-scale A2 plant. Sci Rep 10:6809
Petrovski S, Dyson ZA, Quill ES, McIlroy SJ, Tillett D, Seviour RJ (2011) An examination of the mechanisms for stable foam formation in activated sludge systems. Water Res 45:2146–2154
Pjevac P, Schauberger C, Poghosyan L, Herbold CW, van Kessel MAHJ, Daebeler A, Steinberger M, Jetten MSM, Lucker S, Wagner M, Daims H (2017) AmoA-targeted polymerase chain reaction primers for the specific detection and quantification of Comammox Nitrospira in the environment. Front Microbiol 8:1508
Roots P, Wang YB, Rosenthal AF, Griffin JS, Sabba F, Petrovich M, Yang FH, Kozak JA, Zhang H, Wells GF (2019) Comammox Nitrospira are the dominant ammonia oxidizers in a mainstream low dissolved oxygen nitrification reactor. Water Res 157:396–405
Saad SA, Welles L, Abbas B, Lopez-Vazquez CM, van Loosdrecht MCM, Brdjanovic D (2016) Denitrification of nitrate and nitrite by ‘Candidatus Accumulibacter phosphatis’ clade IC’. Water Res 105:97–109
Sauder LA, Peterse F, Schouten S, Neufeld JD (2012) Low-ammonia niche of ammonia-oxidizing archaea in rotating biological contactors of a municipal wastewater treatment plant. Environ Microbiol 14:2589–2600
Seviour R, Nielsen P (2010) Microbial ecology of activated sludge preface. Microbial Ecology of Activated Sludge: IWA Publishing, London
Seviour RJ, Mino T, Onuki M (2003) The microbiology of biological phosphorus removal in activated sludge systems. FEMS Microbiol Rev 27:99–127
Singleton CM, Petriglieri F, Kristensen JM, Kirkgaard RH, Michaelson TY, Andersen MH, Krondrotaite Z, Karst SM, Dueholm MS, Nielsen PH, Albertsen M (2020) Connecting structure to function with the recovery of over 1000 high-quality activated sludge metagenome-assembled genomes encoding full-length rRNA genes using long-read sequencing. Biorex https://doi.org/10.1101/2020.05.12.088096
Sorokin DY, Lucker S, Vejmelkova D, Kostrikina NA, Kleerebezem R, Rijpstra WIC, Damste JSS, Le Paslier D, Muyzer G, Wagner M, van Loosdrecht MCM, Daims H (2012) Nitrification expanded: discovery, physiology and genomics of a nitrite-oxidizing bacterium from the phylum Chloroflexi. ISME J 6:2245–2256
Speirs LBM, Rice DTF, Petrovski S, Seviour RJ (2019) The phylogeny, biodiversity, and ecology of the Chloroflexi in activated sludge. Front Microbiol 10:2015
Stein LY (2019) Insights into the physiology of ammonia-oxidizing microorganisms. Curr Opin Chem Biol 49:9–15
Subramanian SB, Yan S, Tyagi RD, Surampalli RY (2010) Extracellular polymeric substances (EPS) producing bacterial strains of municipal wastewater sludge: isolation, molecular identification, EPS characterization and performance for sludge settling and dewatering. Water Res 44:2253–2266
Wang MY, Huang GH, Zhao ZR, Dang CY, Liu W, Zheng MS (2018) Newly designed primer pair revealed dominant and diverse comammox amoA gene in full-scale wastewater treatment plants. Bioresour Technol 270:580–587
Wickham H, Wickham Maintainer Hadley (2007) “An implementation of thr grammar of graphics in R.” In “The ggplot package” GPL
Wu L, Ning D, Zhang B, Li Y, Zhang P, Shan X, Zhang Q, Brown M, Li Z, Van Nostrand JD, Ling F, Xiao N, Zhang Y, Vierheilig J, Wells GF, Yang Y, Deng Y, Tu Q, Wang A, Consortium Global Water Microbiome, Zhang T, He Z, Keller J, Nielsen PH, PJJ A, Criddle CS, Wagner M, Tiedje JM, He Q, Curtis TP, Stahl DA, Alvarez-Cohen L, Rittmann BE, Wen X, Zhou J (2019) Global diversity and biogeography of bacterial communities in wastewater treatment plants. Nat Microbiol 4:1183–1195
Xia Y, Kong YH, Thomsen TR, Nielsen PH (2008) Identification and ecophysiological characterization of epiphytic protein-hydrolyzing Saprospiraceae (“Candidatus epiflobacter” spp.) in activated sludge’. Appl Environ Microbiol 74:2229–2238
Xia Y, Hu M, Wen XH, Wang XH, Yang YF, Zhou JZ (2016) Diversity and interactions of microbial functional genes under differing environmental conditions: insights from a membrane bioreactor and an oxidation ditch. Sci Rep 6:18509
Xia Y, Wen XH, Zhang B, Yang YF (2018) Diversity and assembly patterns of activated sludge microbial communities: a review. Biotechnol Adv 36:1038–1047
Ye L, Zhang T (2013) Bacterial communities in different sections of a municipal wastewater treatment plant revealed by 16S rDNA 454 pyrosequencing. Appl Microbiol Biotechnol 97:2681–2690
Zhang Y, Chen LJ, Sun RH, Dai TJ, Tian JP, Wen DH (2015) Ammonia-oxidizing bacteria and archaea in wastewater treatment plant sludge and nearby coastal sediment in an industrial area in China. Appl Microbiol Biotechnol 99:4495–4507
Zhang B, Ning DL, Yang YF, Van Nostrand JD, Zhou JZ, Wen XH (2020) Biodegradability of wastewater determines microbial assembly mechanisms in full-scale wastewater treatment plants. Water Res 169:115276
Zhao D, Huang R, Zeng J, Yu Z, Liu P, Cheng S, Wu QL (2014) Pyrosequencing analysis of bacterial community and assembly in activated sludge samples from different geographic regions in China. Appl Microbiol Biotechnol 98:9119–9128
Zhao ZR, Huang GH, He SS, Zhou N, Wang MY, Dang CY, Wang JW, Zheng MS (2019) Abundance and community composition of comammox bacteria in different ecosystems by a universal primer set. Sci Total Environ 691:146–155
Zheng MS, Wang MY, Zhao ZR, Zhou N, He SS, Liu SF, Wang JW, Wang XK (2019) Transcriptional activity and diversity of comammox bacteria as a previously overlooked ammonia oxidizing prokaryote in full-scale wastewater treatment plants. Sci Total Environ 656:717–722
Acknowledgements
We wish to thank Peter Griffith for his insights into the operational features of these plants.
Funding
This work was supported by the La Trobe University Research Focus Area, Securing Food, Water and Environment. Daniel Rice was supported by an LTU postgraduate scholarship.
Author information
Authors and Affiliations
Contributions
SP, DT, and RS conceived and designed the experiment; SP, DT, and SB conducted the experiments; DT and TN performed the data analysis; and RS wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 1130 kb)
Rights and permissions
About this article
Cite this article
Petrovski, S., Rice, D.T.F., Batinovic, S. et al. The community compositions of three nitrogen removal wastewater treatment plants of different configurations in Victoria, Australia, over a 12-month operational period. Appl Microbiol Biotechnol 104, 9839–9852 (2020). https://doi.org/10.1007/s00253-020-10901-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10901-8