Abstract
Various animal models are used in the study of alcoholism, with the honeybee (Apis mellifera L.) among them. Here, we tested the hypothesis that foragers show higher intoxication resistance to alcohol than nurses, an issue thus far not investigated. To this end, we measured the latency to full sedation when exposed to alcohol in foragers, nurses and reverted nurses. In addition, we measured alcohol dehydrogenase (ADH) levels in these worker castes. Caste status was confirmed by comparison of the size of their hypopharyngeal glands. We detected high intoxication resistance to alcohol and presence of ADH in foragers. In nurses, we detected significantly lower intoxication resistance to alcohol and no ADH. These between-caste differences cannot be explained by the age difference between castes as in reverted nurses, characterized by similar age to foragers, we detected an intermediate intoxication resistance to alcohol and no ADH. Our results suggest possible natural exposure to alcohol in different castes of workers. As such, we further develop the honeybee as a model in alcoholism-related research and open new research avenues.
Similar content being viewed by others
1 Introduction
In humans, the possibility of abusive alcohol consumption is high as the availability of alcoholic beverages is widespread. Various animal models are used in order to conduct studies related to alcoholism. Studies on the neurological and behavioural effects of alcohol consumption as well as on the development of diseases related to alcoholism are usually performed on mammals, but invertebrate models have been successfully developed and used for many years. These models primarily include Caenorhabditis worms and Drosophila flies (Scholz and Mustard 2011; Søvik and Barron 2013). The use of these invertebrate models is well justified as many effects of alcohol appear conserved across animal phyla (Wolf and Heberlein 2003). Nevertheless, a number of issues are not feasible or are difficult to study when using worms or flies, such as cognitive or social behaviour. There is another organism, recently introduced into the field, which bypasses at least some of these issues. The honeybee (Apis mellifera L.) is already an established model in many biological areas of research. It possesses some traits common to Caenorhabditis or Drosophila, such as well-known physiology or neuroanatomy, and possesses several distinctive traits, including outstanding cognitive abilities and advanced social behaviour (Winston 1987). These latter traits make the honeybee especially valuable in the context of alcoholism-related research.
Several studies have already made a good case in establishing the honeybee as a model in research devoted to alcoholism. It has been demonstrated that bees, which readily consumed up to 20% ethyl alcohol solution with sucrose, displayed no significant aversion or avoidance to this drug and even preferred solutions containing it (Abramson et al. 2000, 2004a; Sokolowski et al. 2012; Varnon et al. 2018; Mustard et al. 2019). Furthermore, ethanol consumption by bees was showed to result in dose-dependent impairment of feeding activity, locomotion, social behaviour and learning (Abramson et al. 2000, 2004b, 2005, 2015; Božič et al. 2006; Maze et al. 2006; Mustard et al. 2008; Mixson et al. 2010; Wright et al. 2012; Giannoni-Guzmán et al. 2014). These post-exposure behavioural changes parallel those observed in mammals, including humans. Other studies have gone as far as blocking proneness to consume ethanol in bees using Antabuse®, a drug developed to treat human alcoholics (Abramson et al. 2003). Also, the tolerance effect to ethanol, a hallmark of alcoholism, was recently demonstrated in the honeybee, in the form of a drop in the impairing effect of ethanol on locomotion after repeated exposure to the drug (Miler et al. 2018). However, even in light of these studies, our knowledge of the natural history of the honeybee with regard to ethanol exposure remains rudimentary and hinders the development of the honeybee as a model in research devoted to alcoholism.
The natural consumption rates of ethanol by bees remain unknown, but it seems likely that they must encounter ethanol under natural conditions. Floral nectar, their major energy source, is often corrupted with nectarivorous yeasts (Herrera et al. 2008; de Vega et al. 2009) and may ferment to produce ethanol, even if only in low concentrations (Kevan et al. 1988; Jakubska et al. 2005; Goodrich et al. 2006; Wiens et al. 2008). Furthermore, food stored inside of the hive, especially freshly stored food which is not yet concentrated, under appropriate conditions may also ferment to produce ethanol (Gilliam 1979). Moreover, the honeybee possesses alcohol dehydrogenase (ADH), one of the major enzymes for ethanol breakdown during digestion (Martins et al. 1977; Bouga et al. 2005), and the workers need to ingest pure ethanol in order to biosynthesize an important pheromone, ethyl oleate, responsible for maintaining the division of labour in the colony (Leoncini et al. 2004; Castillo et al. 2012a, 2012b). The existence of an ethanol breakdown metabolic pathway, as well as ethanol-dependent regulatory mechanism of the division of labour, speaks for the exposure of the honeybee to ethanol both in the past and in the present.
The aim of this study was to provide additional support in favour of the honeybee being an appropriate model in alcoholism-related research by investigating the intoxication resistance, expressed as the latency to full sedation when exposed to ethanol, in different honeybee worker castes. The probabilities of potential encounter with ethanol likely differ between these castes and reflect on their intoxication resistance. As already mentioned, the honeybee displays division of labour, with some individuals, the so-called nurses, performing in-hive tasks, and some others, the so-called foragers, working outside of the hive (Winston 1987). Nurses stay inside the nest and consequently can only encounter ethanol when stored food undergoes fermentation. Foragers leave the nest and collect resources, meaning that they might come in contact with ethanol directly in the field. Therefore, in nurses, ethanol exposure is passive and likely rare, whereas in foragers it is more active and likely frequent. Using this assumption and taking into account the finding that workers display a tolerance effect to ethanol when they encounter it repeatedly (Miler et al. 2018), we hypothesised that foragers would show higher intoxication resistance to ethanol than nurses. However, there is an issue connected to the proper testing of this hypothesis, namely that the division of labour which occurs in the honeybee is heavily age-dependent.
Nurses and foragers differ not only in the type of task they perform, but also in age (Jeanne 1985; Woyciechowski and Kozłowski 1998; Tofilski 2009). Younger bees perform nurse work and older bees transition into foragers. One way of partially distinguishing between these effects lies in a phenomenon termed reversion (Amdam et al. 2005; Kuszewska and Woyciechowski 2013). When the colony demography is suddenly disrupted (i.e. drop in the number of nurses), some foragers show reversion and transition back into workers performing nurse like in-hive tasks. Reversion is more likely to occur in the youngest foragers (Robinson et al. 1992; Page Jr et al. 1992). Nevertheless, in terms of age, reverted nurses are probably more similar to foragers than nurses, especially considering that the process of reversion is highly plastic and even old foragers are known to revert (Huang and Robinson 1996; Amdam et al. 2005; Kuszewska and Woyciechowski 2013). Here, to test our hypothesis, we used all three of these worker castes. We predicted foragers to show the best intoxication resistance, with the worst results in nurses and intermediate resistance in reverted nurses. In order to confirm the caste status of workers, we measured the size of their hypopharyngeal glands (HPG), which differentiates nurses and foragers. Hypertrophy of these glands in nurses enables the production of brood food and increases capacity for brood care activity (Huang and Otis 1989; Hrassnigg and Crailsheim 1998). Reverted nurses should display hypertrophy alike nurses in order to be able to perform their in-hive tasks (Huang and Robinson 1996; Amdam et al. 2005; Kuszewska and Woyciechowski 2013). In addition to the behavioural measure of the intoxication resistance, expressed as the latency to full sedation when exposed to ethanol, we analysed the gene expression and protein levels of ADH in each caste. We predicted it both to mirror the behavioural characteristics, i.e. to be high in foragers, low in nurses and intermediate in reverted nurses.
To sum up, here, we tested the hypothesis that foragers show higher intoxication resistance to ethanol than nurses. To do this, we investigated the latency to full sedation when exposed to ethanol and the gene expression and protein levels of ADH in nurses, foragers and reverted nurses. To confirm the status of these worker castes, we used an anatomical parameter, i.e. the size of their HPG. Our predictions were tentatively based on the possible natural exposure to ethanol of the different castes, as described above. Alternatively, we considered such exposure to be null, in which case the pattern of responses to ethanol should rest entirely on the age-dependent body state. To allow for this alternative to be detected, we tested intoxication resistance not only to ethyl alcohol but also to ethyl acetate, which similarly as ethanol results in sedation. We expected that nurses would show the greatest resistance, whereas foragers and reverted nurses would exhibit similar low resistance.
2 Material and methods
The experiment was conducted in June 2019, over a week-long period. Three different honeybee colonies were used and the experiment was performed on one colony at a time (two days devoted for a single colony). In each colony, bees from different castes were obtained by means of hive manipulation. All relevant apiary equipment (hives, frames, etc.) was manufactured by Łysoń (Poland).
2.1 Worker collection
In the early morning, the original hive with the selected colony was moved from its usual location in the apiary and a new hive was put in its place. In the new hive, several bee-free frames with uncapped and capped brood and food from the original hive were present. This manipulation ensured that all active foragers leaving the now relocated hive for their trips, based on their memory, would return to the new hive, leaving only nurses in the original hive. Thus, around noon, it was possible to collect nurses and foragers. Nurses were taken from a randomly selected brood frame obtained from the original hive whereas foragers were captured at the entrance to the new hive. Nurses and foragers were kept in separate experimental cages and then held in an incubator (Pol-Eco Aparatura, Poland) at 32 °C for an hour with ad libitum sucrose solution and water. The new hive, now filled with foragers after their trips, was left to overnight. Early the next morning, this hive was moved from its designated place in the apiary and an empty hive was put in its place. This manipulation ensured that all active foragers leaving for their trips, based on their memory, would return to the empty hive, leaving only reverted nurses in the new hive. Thus, around noon, it was possible to collect reverted nurses. Reverted nurses were taken from a randomly selected brood frame from the new hive, kept in an experimental cage and then held in an incubator (Pol-Eco Aparatura, Poland) at 32 °C for an hour with ad libitum sucrose solution and water. The number of collected nurses, reverted nurses and foragers was about 150 each per colony. Out of this number, 80 were designated for behavioural testing, 10 were designated for ADH gene expression and protein level analyses, and 10 were designated for HPG dissection and measurement.
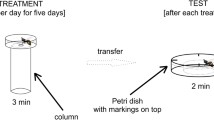
2.2 Behavioural testing
After the hour-long acclimation period, the experimental cage with workers (nurses, foragers, reverted nurses) was taken out of the incubator. Of the available alive workers inside the cage, the 20 designated for other purposes (ADH, HPG) were placed individually into 1.5 ml tubes (Eppendorph, USA), and then frozen at – 80 °C, until needed, in an ultralow temperature freezer (Haier Bio-Medical, China). For the remaining living workers, half (40 bees) were exposed to fumes of ethyl acetate (control substance) whereas the remaining half were exposed to fumes of ethyl alcohol (experimental substance). Substances were obtained from Sigma-Aldrich (USA). In each test, a single bee was individually enclosed inside a transparent Petri dish (3.5 cm in diameter) with a hole (0.5 cm in diameter) in the bottom. The dish was then placed on a column (3.0 cm in diameter) filled with 30 ml of room temperature 98% ethyl acetate or 98% ethyl alcohol. Tests for both substances were conducted simultaneously. Intoxication resistance, i.e. the latency to full sedation, was measured for each bee. Statistical analysis was performed using the statistical programming language R (R Core Team 2020) with the lme4, lmerTest, emmeans and ggpubr packages. Using the linear mixed model by maximum likelihood, we analysed how the latency to full sedation when exposed to ethyl acetate and ethyl alcohol depends on caste (nurse vs reverted nurse vs forager; fixed effect) and colony (first vs second vs third; random effect). Results of the specific comparisons between castes provided post-hoc Tukey test.
2.3 Alcohol dehydrogenase gene expression analysis
Out of the 20 frozen individuals from each caste and colony, 10 were used for analysing their ADH gene expression. Each bee was first homogenized and then a part of the homogenate was used for the analysis. Total RNA was extracted from each individual with TRIzol reagent (Life Technologies, USA) according to the manufacturer’s instructions. To remove contaminating DNA and DNase from RNA preparations, TURBO DNase-free Kit was used (Ambion, USA). The yield and quality of the RNA were assessed by measuring the A260:A280 ratio in a NanoDrop ND2000 Spectrophotometer (Thermo Scientific, USA) and by electrophoresis. The purified total RNA was used to generate total cDNA using high-capacity cDNA Reverse Transcription Kit (Applied Biosystems, USA) according to the manufacturer’s protocol. Total cDNA was prepared in a 20-μl volume using the random primers, dNTPmix, RNAse inhibitor and reverse transcriptase (RT). For a negative control, the same reactions without adding of RT were performed simultaneously (1 μl of RNase-free water was added in place of RT). The RT+ and RT− samples were then subjected to PCR amplification performed in a Veriti Thermal Cycler (Applied Biosystems, USA). Real-time RT-PCR was performed using the StepOne Real-Time PCR system (Applied Biosystems, USA) and optimized standard conditions as described above. Primer sets (Institute of Biochemistry and Biophysics, Polish Academy of Sciences, Warsaw, Poland), designed de novo, are listed in Table I. Amplification efficiency was determined as described by Svec and others (Svec et al. 2015). All PCR assays displayed efficiency between 90 and 115%. Detection of amplification products for individual genes was performed with 10 ng cDNA, 0.5 mM primers and SYBR Green master mix (Applied Biosystems, USA) in a final volume of 20 μl. Amplifications were performed as follows: 55 °C for 2 min, 94 °C for 10 min, followed by annealing temperature for 30 and 45 s 72 °C to determine the cycle threshold (Ct) for quantitative measurement. To confirm amplification specificity, the PCR products from each primer pair were subjected to melting curve analysis and subsequent agarose gel electrophoresis. Relative quantification (RQ) was obtained using the 2∆∆ method, adjusting the ADH mRNA expression to the expression of β-actin mRNA and taking the adjusted expression in the foragers’ tissue samples as reference (RQ = 1) (Livak and Schmittgen 2001). Three independent experiments were performed, each in triplicate. All PCR products were analysed by gel electrophoresis on 1.5–2.5% agarose gels with ethidium bromide together with a ready-to-load 100-bp DNA ladder marker (Promega, UK) and followed by fluorescence digitisation using a Bio-Rad GelDoc XR system (Bio-Rad Labs, USA). Since there were no differences between colonies, the mean RQ of all examined colonies was compared (see Supplementary Fig. 1). The purpose of this RT-qPCR analysis was to check which specific type of ADH was expressed in our samples (anti-alcohol dehydrogenase type I (ADH1), ADH2, ADH3). Due to the considerable conservation of the ADH genes within Animalia, we additionally checked Drosophila ADH.
2.4 Alcohol dehydrogenase level analysis
The remaining part of the earlier prepared homogenate was used for analysing ADH levels by means of Western blotting. The focus here was solely on the ADH1 protein as it was expressed in our samples (see above and Section 3). The proteins were extracted from each individual with a cold RIPA buffer (Thermo-Fisher, USA) supplemented with protease inhibitors (Thermo-Fisher, USA), sonicated and centrifuged at 10000g for 20 min at 4 °C. Then, pooled samples were prepared, each by mixing 30 μl of the extraction from individuals belonging to a given caste and colony (3 castes × 3 colonies = 9 pooled samples). Sample pooling was undertaken in order to reduce the effects of individual variation and to show representative results of group changes (Warrington et al. 2000). Pooled samples were prepared 5 times to decrease chances for procedural errors and false results (9 samples × 5 times = 45 pooled samples in total). The protein concentration in all of these pooled samples was estimated by the Bio-Rad DC Protein Assay Kit with BSA as a standard (Bio-Rad Laboratories, USA): 100 μg of each pooled sample was solubilised in a sample buffer (Bio-Rad Laboratories, USA) and heated at 99.9 °C for 5 min. Proteins were then separated by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS–PAGE) on 10% (vol/vol) polyacrylamide gels under reducing conditions and transferred onto a polyvinylidene difluoride membranes (Merck Millipore, USA). Nonspecific binding sites were blocked with a solution of 5% (wt/v) non-fat, dry milk containing 0.1% (v/v) Tween® 20. Next, the membranes were incubated with the ADH1 antibody no. ab80354 (1:1000; Abcam, UK) at 4 °C overnight, followed by a goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:3000; Vector Laboratories, USA) for 1 h at room temperature. Immunoreactive proteins were detected by chemiluminescence and images were captured with a ChemiDocTM XRS+ System (Bio-Rad Laboratories, USA). All immunoblots were stripped with buffer containing 62.5 mM Tris- HCL, 100 mM 2-mercaptoethanol and 2% SDS (wt/vol; pH 6.7) at 50 °C for 30 min and incubated in an antibody against β-actin (1:2000; Sigma-Aldrich, USA), which served as the loading control. The molecular weights of target proteins were estimated by reference to standard proteins (Sigma-Aldrich, USA). To obtain quantitative results, immunoblots were analysed densitometrically using the ImageLab software (Bio-Rad Laboratories, USA). Each data point was normalized against its corresponding β-actin data point, resulting in the relative optical density (ROD) measure for each band. Mean ± standard deviation of ROD was then calculated for each of the 9 types of the pooled samples (3 castes × 3 colonies).
2.5 Hypopharyngeal gland dissection and measurement
Of the 20 frozen individuals from each caste and colony, 10 were used for dissecting and measuring their HPG. Frozen workers were dissected, stained with Giemsa reagent (Stamar, Poland) for approximately 10 s, rinsed and then examined under a stereomicroscope (Nicon H550L, Japan). The size of the HPG was calculated from the average diameter of 10 acini [square root of the longest × shortest diameters of 5 acini from the right gland and 5 from the left gland; the HPG consists of a great number of lobes, called acini, and their diameter is used routinely as an index of the gland size (Wegener et al. 2009)]. Statistical analysis was similar to that for the behavioural data: we modelled how the mean acini diameter depends on caste (fixed effect) and colony (random effect) using linear mixed model (R Core Team 2020).
3 Results
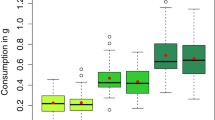
The intoxication resistance depended on caste for ethyl acetate (F2,357 = 113.53, p < 0.001, highest resistance in nurses, Figure 1) as well as ethyl alcohol (F2,357 = 18.82, p < 0.001, highest resistance in foragers, Figure 2). In both ethyl acetate and ethyl alcohol, the colony factor explained very little (less than 1%) of the variance unexplained by caste (χ2 = 1.29, p = 0.256 in the ethyl acetate model and χ2 = 0.21, p = 0.646 in the ethyl alcohol model). In terms of the ADH analyses, only ADH1, analogous to Drosophila ADH, was expressed in the tested material and it was detectable only in foragers (Table II, see also Supplementary Figure 1 and 2 for gel pictures and additional data). In nurses as well as reverted nurses, there were no bands in the PCR and blot; thus ROD values were close to null. Caste differences in the size of HPG were confirmed as predicted, with nurses and reverted nurses having higher mean acini diameters than foragers (F2,87 = 256.58, p < 0.001, Figure 3). The colony factor, as in the case of intoxication resistance, was non-significant (χ2 = 0.08, p = 0.776).
The intoxication resistance to ethyl acetate, expressed as the latency to full sedation, in workers belonging to different castes. Figure shows data pooled together for the three used colonies. The number of tested bees in each caste is N = 120. Lines inside squares indicate means, squares indicate quartiles and whiskers indicate ranges. Statistical significance as yielded by the Tukey post-hoc comparisons.
The intoxication resistance to ethyl alcohol, expressed as the latency to full sedation, in workers belonging to different castes. Figure shows data pooled together for the three used colonies. The number of tested bees in each caste is N = 120. Lines inside squares indicate means, squares indicate quartiles and whiskers indicate ranges. Statistical significance as yielded by the Tukey post-hoc comparisons.
The HPG size, expressed as the mean acini diameter, in workers belonging to different castes. Figure shows data pooled together for the three used colonies. The number of tested bees in each caste is N = 30. Lines inside squares indicate means, squares indicate quartiles and whiskers indicate ranges. Statistical significance as yielded by the Tukey post-hoc comparisons.
4 Discussion
In the present study, our key focus was on the intoxication resistance to ethanol in nurses, foragers and reverted nurses. The observed pattern indicated that foragers were most resistant to intoxication, nurses were least resistant and reverted nurses exhibited intermediate results. For ethyl acetate we showed that the pattern of the intoxication resistance was clearly age-dependent, with the youngest nurses found to be more resistant than both older castes, reverted nurses and foragers. This general age-dependent pattern of stress resistance (Remolina et al. 2007), visible for ethyl acetate, strongly suggests that our reverted nurses and foragers were similar in terms of age. As this is contrary to some earlier studies showing that reversion was more likely to occur in the youngest foragers (Robinson et al. 1992; Page Jr et al. 1992; Kuszewska and Woyciechowski 2013), the present results should be treated carefully. Furthermore, it is advisable for future studies on the intoxication resistance to ethanol to make use of other ways of controlling age, such as tests of cohorts of workers and/or precocious foragers. Nevertheless, our present results on the intoxication resistance to ethanol seem unlikely explained by the age difference between castes. In our opinion, high resistance to ethanol in foraging workers points more towards their adaptation to an increased encounter rate with ethanol.
Indeed, foragers were recently demonstrated to prefer ethanol consumption in a choice assay between food containing 2.5% ethanol and that free of ethanol (Mustard et al. 2019). Honeybees may therefore possess sensory biases towards identification and consumption of ethanol, and like some other invertebrates, use ethanol as a locational cue for identification of transient and valuable food sources (Dierks and Fischer 2008). Furthermore, ethyl oleate, a pheromone responsible for maintaining the division of labour within a colony, is synthetized only by foragers from pure collected ethanol, which means that they necessarily need to encounter this drug (Leoncini et al. 2004; Castillo et al. 2012a, 2012b). Overall, our results most likely stem from differences in task performance and thus natural exposure to ethanol in intranidal and extranidal workers. Similar lines of interpretation were used in the case of Drosophila. Specifically, only young reproducing flies are naturally exposed to ethanol and, accordingly, ageing was shown to decrease their ethanol resistance (De Nobrega and Lyons 2016; De Nobrega et al. 2017).
Our complementary results of resistance to ethanol, i.e. ADH gene expression and protein levels in different castes, were in line with the behavioural characteristics. No detectable ADH in nurses and the presence of this protein in foragers fit the natural exposure interpretation. In the future, a thorough analysis of the expression profiles of alcoholism-related genes in workers belonging to different castes is in order, similarly to that performed, e.g. for aggression in young and old bees (Alaux et al. 2009). Somewhat surprisingly, however, we detected no ADH in reverted nurses. This suggests profound changes happening quickly during reversion, with the herein reported modification of HPG size being one example, and perhaps alterations of ADH levels being another. However, it is important to note that the anatomical, behavioural and physiological changes in reverting workers surely occur with varying rapidness (for demonstration of progressive changes see e.g. Huang and Robinson 1996 and Amdam et al. 2005). A lack of ADH in reverted nurses may alternatively stem from the function of this protein being not fully connected to our chosen behavioural measure of intoxication resistance. Here, we exposed workers to ethanol fumes. The main advantage of using exposition to ethanol fumes instead of ethanol ingestion lies in bypassing the metabolic side effects of digestion, which is especially complicated in the honeybee because of the presence of the crop, a specialised part of the foregut (Winston 1987). By using fumes, the drug enters the workers via the tracheal system and not the digestive system (Ammons and Hunt 2008). The levels of ADH might therefore be connected to digestion of ethanol rather than to counteract ethanol-induced sedation. Such an explanation may account for why, in Drosophila, ethanol breakdown capabilities only partly explain ethanol poisoning resistance (Fry 2014).
Consumption of ethanol in the field can be both beneficial as well as detrimental for honeybees. In Drosophila, ethanol consumption was demonstrated to decrease parasitic load (Milan et al. 2012; Lynch et al. 2016). Similar effects may occur in the honeybee, e.g. Nosema infection rates may drop in foragers consuming ethanol. The beneficial influence of consumed food on the risk of Nosema infection was demonstrated in a study reporting on the importance of fungicide presence in pollen (Pettis et al. 2013). On the other hand, consuming ethanol-corrupted sugar may disrupt homing ability of foragers and cause them to lose their way to the hive (Abramson et al. 2000, 2004a, 2005; Maze et al. 2006; Sokolowski et al. 2012). Of particular importance, in this context, is the development of tolerance in workers (Miler et al. 2018), which may act to reduce the magnitude of any detrimental effects.
In summary, our hypothesis that foragers show greater intoxication resistance to ethanol than nurses was confirmed. This effect is most likely connected to increased ethanol exposure in foragers than in nurses and not to their greater age. We encourage further studies into the natural history of the honeybee in terms of ethanol exposure.
References
Abramson CI, Stone SM, Ortez RA, Luccardi A, Vann KL, Hanig KD, Rice J (2000) Development of an ethanol model using social insects I: behaviour studies of the honey bee (Apis mellifera L.). Alcohol Clin Exp Res 24:1153-1166. https://doi.org/10.1111/j.1530-0277.2000.tb02078.x.
Abramson CI, Fellows GW, Browne BL, Lawson A, Ortiz RA (2003) Development of an ethanol model using social insects II: effect of Antabuse® on consumatory responses and learned behaviour of the honey bee (Apis mellifera L.). Psychol Rep 92:365-378. https://doi.org/10.2466/pr0.2003.92.2.365.
Abramson CI, Kandolf A, Sheridan A, Donohue D, Božič J, Meyers JE, Benbassat D (2004a) Development of an ethanol model using social insects III: preferences for ethanol solutions. Psychol Rep 94:227-239. https://doi.org/10.2466/pr0.94.1.227-239.
Abramson CI, Place AJ, Aquino IS, Fernandez A (2004b) Development of an ethanol model using social insects IV: influence of ethanol on the aggression of Africanized honey bees (Apis mellifera L.). Psychol Rep 94:1107-1115. https://doi.org/10.2466/pr0.94.3c.1107-1115.
Abramson CI, Sanderson C, Painter J, Barnett S, Wells H (2005) Development of an ethanol model using social insects V: honeybee foraging decisions under the influence of alcohol. Alcohol 36:187-193. https://doi.org/10.1016/j.alcohol.2005.09.001.
Abramson CI, Craig DPA, Varnon CA, Wells H (2015) The effect of ethanol on reversal learning in honey bees (Apis mellifera anatolica): response inhibition in a social insect model. Alcohol 49:245-258. https://doi.org/10.1016/j.alcohol.2015.02.005.
Alaux C, Sinha S, Hasadsri L, Hunt GJ, Guzmán-Novoa E, DeGrandi-Hoffman G, Uribe-Rubio JL, Southey BR, Rodriguez-Zas S, Robinson GE (2009) Honey bee aggression supports a link between gene regulation and behavioral evolution. PNAS 106:15400-15405. https://doi.org/10.1073/pnas.0907043106.
Amdam GV, Aase ALTO, Seehuus S-T, Fondrk MK, Norberg K, Hartfelder K (2005) Social reversal of immunosenescence in honey bee workers. Exp Gerontol 40:939-947. https://doi.org/10.1016/j.exger.2005.08.004.
Ammons AD, Hunt GJ (2008) Characterization of honey bee sensitivity to ethanol vapour and its correlation with aggression. Alcohol 42:129-136. https://doi.org/10.1016/j.alcohol.2007.12.005.
Bouga M, Kilias G, Harizanis PC, Papasotiropoulos V, Alahiotis S (2005) Allozyme variability and phylogenetic relationships in honey bee (Hymenoptera: Apidae: Apis mellifera) populations from Greece and Cyprus. Biochem Genet 43:471-483. https://doi.org/10.1007/s10528-005-8163-2.
Božič J, Abramson CI, Bedencic M (2006) Reduced ability of ethanol drinkers for social communication in honeybees (Apis mellifera carnica Poll.). Alcohol 38:179-183. https://doi.org/10.1016/j.alcohol.2006.01.005.
Castillo C, Maisonnasse A, Le Conte Y, Plettner E (2012a) Seasonal variation in the titers and biosynthesis of the primer pheromone ethyl oleate in honey bees. J Insect Physiol 58:1112-1121. https://doi.org/10.1016/j.jinsphys.2012.05.010.
Castillo C, Chen H, Graves C, Maisonnasse A, Le Conte Y, Plettner E (2012b) Biosynthesis of ethyl oleate, a primer pheromone, in the honey bee (Apis mellifera L.). Insect Biochem Mol Biol 42:404-416. https://doi.org/10.1016/j.ibmb.2012.02.002.
De Nobrega AK, Lyons IC (2016) Circadian modulation of alcohol-induced sedation and recovery in male and female Drosophila. J Biol Rhytm 31:142-160. https://doi.org/10.1177/0748730415627067.
De Nobrega AK, Mellers AP, Lyons LC (2017) Aging and circadian dysfunction increase alcohol sensitivity and exacerbate mortality in Drosophila melanogaster. Exp Ger 97:49-59. https://doi.org/10.1016/j.exger.2017.07.014.
Dierks A, Fischer K (2008) Feeding responses and food preferences in the tropical, fruit-feeding butterfly, Bicyclus anynana. J Insect Physiol 54:1363-1370. https://doi.org/10.1016/j.jinsphys.2008.07.008.
Fry JD (2014) Mechanisms of naturally evolved ethanol resistance in Drosophila melanogaster. J Exp Biol 217:3996-4003. https://doi.org/10.1242/jeb.110510.
Giannoni-Guzmán MA, Giray T, Agosto-Rivera JL, Stevison BK, Freeman B, Ricci P, Brown EA, Abramson CI (2014) Ethanol-induced effects on sting extension response and punishment learning in the Western honey bee (Apis mellifera). PLOS ONE 9:e100894. https://doi.org/10.1371/journal.pone.0100894.
Gilliam M (1979) Microbiology of pollen and bee bread – the yeasts. Apidologie 10:43-53. https://doi.org/10.1051/apido:19790106.
Goodrich KR, Zjhra ML, Ley CA, Raguso RA (2006) When flowers smell fermented: the chemistry and ontogeny of yeasty floral scent in pawpaw (Asimina triloba, Annonaceae). Int J Plant Sci 167:33-46. https://doi.org/10.1086/498351.
Herrera CM, Garcia IM, Perez R (2008) Invisible floral larcenies: microbial communities degrade floral nectar of bumble bee-pollinated plants. Ecology 89:2369-2376. https://doi.org/10.1890/08-0241.1.
Hrassnigg N, Crailsheim K (1998) Adaptation of hypopharyngeal gland development to the brood status of honeybee (Apis mellifera L.) colonies. J Insect Physiol 44:929-939. https://doi.org/10.1016/S0022-1910(98)00058-4.
Huang Z-Y, Otis G (1989). Factors determining hypopharyngeal gland activity of worker honey bees (Apis mellifera L.). Insect Soc 36:264-276. https://doi.org/10.1007/BF02224880.
Huang Z-Y, Robinson GE (1996) Regulation of honey bee division of labor by colony age demography. Behav Ecol Sociobiol 39:147-158. https://doi.org/10.1007/s002650050276.
Jakubska A, Prządo D, Steininger M, Anioł-Kwiatkowska J, Kadej M (2005) Why do pollinators become “sluggish”? Nectar chemical constituents from Epipactis helleborine (L.) Crantz (Orchidaceae). App Ecol Env Res 3:29-38. https://doi.org/10.15666/aeer/0302_029038.
Jeanne RL (1985) The evolution of the organization of work in social insects. Monit Zoo Ital 20:119-133. https://doi.org/10.1080/00269786.1986.10736494.
Kevan PG, Eisikowitch D, Fowle S, Thomas K (1988) Yeast-contaminated nectar and its effects on bee foraging. J Api Res 27:26-29. https://doi.org/10.1080/00218839.1988.11100777.
Kuszewska K, Woyciechowski M (2013) Reversion in honeybee, Apis mellifera, workers with different life expectancies. Anim Behav 85:247-253. https://doi.org/10.1016/j.anbehav.2012.10.033.
Leoncini I, Le Conte Y, Castagliola G, Plettner E, Toth AL, Wang M, Huang Z, Becard J-M, Crauser D, Slessor KN, Robinson GE (2004) Regulation of behavioral maturation by a primer pheromone produced by adult worker honey bees. PNAS 101:17559-17564. https://doi.org/10.1073/pnas.0407652101.
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Methods 25:402-408. https://doi.org/10.1006/meth.2001.1262.
Lynch ZR, Schlenke TA, Morran LT, de Roode JC (2016) Ethanol confers differential protection against generalist and specialist parasitoids of Drosophila melanogaster. PLOS ONE 12:e0180182. https://doi.org/10.1371/journal.pone.0180182.
Martins E, Mestriner MA, Contel EPB (1977) Alcohol dehydrogenase polymorphism in Apis mellifera. Biochem Genet 15:357-366. https://doi.org/10.1007/BF00484466.
Maze IS, Wright GA, Mustard JA (2006) Acute ethanol ingestion produces dose-dependent effects on motor behaviour in the honey bee (Apis mellifera). J Insect Physiol 52:1243-1253. https://doi.org/10.1016/j.jinsphys.2006.09.006.
Milan NF, Kacsoh BZ, Schlenke TA (2012) Alcohol consumption as self-medication against blood-borne parasites in the fruit fly. Curr Biol 22:488-493. https://doi.org/10.1016/j.cub.2012.01.045.
Miler K, Kuszewska K, Privalova V, Woyciechowski M (2018) Honeybees show adaptive reactions to ethanol exposure. Sci Rep 8:8707. https://doi.org/10.1038/s41598-018-27117-6.
Mixson TA, Abramson CI, Božič J (2010) The behavior and social communication of honey bees (Apis mellifera carnica Poll.) under the influence of alcohol. Psychol Rep 106:701-707. https://doi.org/10.2466/pr0.106.3.701-717.
Mustard JA, Edgar EA, Mazade RE, Wu C, Lillvis JL, Wright GA (2008) Acute ethanol ingestion impairs appetitive olfactory learning and odor discrimination in the honey bee. Neurobiol Learn Mem 90:633-643. https://doi.org/10.1016/j.nlm.2008.07.017.
Mustard JA, Oquita R, Garza P, Stoker A (2019) Honey bees (Apis mellifera) show a preference for the consumption of ethanol. Alcohol Clin Exp Res 43:26-35. https://doi.org/10.1111/acer.13908.
Page RE Jr, Robinson GE, Britton DS, Fondrk MK (1992) Genotypic variability for rates of behavioral development in worker honeybees (Apis mellifera L.). Behav Ecol 3:173-180. https://doi.org/10.1093/beheco/3.2.173.
Pettis JS, Lichtenberg EM, Andree M, Stitzinger J, Rose R, van Engelsdorp D (2013) Crop pollination exposes honey bees to pesticides which alter their susceptibility to the gut pathogen Nosema ceranae. PLOS ONE 8:e70182. https://doi.org/10.1371/journal.pone/0070182.
R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org
Remolina SC, Hefez DM, Robinson GE, Hughes KA (2007) Senescence in the worker honey bee Apis mellifera. J Insect Physiol 53:1027-1033. https://doi.org/10.1016/j.insphys.2007.05.015.
Robinson GE, Page RE Jr, Strambi C, Strambi A (1992) Colony integration in honey bees: mechanisms of behavioral reversion. Ethology 90:336-348. https://doi.org/10.1111/j.1439-0310.1992.tb00844.x.
Scholz H, Mustard JA (2011) Invertebrate models of alcoholism. Curr Topics Behav Neurosci 13:433-457. https://doi.org/10.1007/7854_2011_128.
Sokolowski MBC, Abramson CI, Craig DPA (2012) Ethanol self-administration in free-flying honeybees (Apis mellifera L.) in an operant conditioning protocol. Alcohol Clinic Exp Res 36:1568-1577. https://doi.org/10.1111/j.1530-0277.2012.01770.x.
Søvik E, Barron AB (2013) Invertebrate models in addiction research. Brain Behav Evol 82:153-165. https://doi.org/10.1159/000355506.
Svec D, Tichopad A, Novosadova V, Pfaffl MW, Kubista M (2015) How good is a PCR efficiency estimate: recommendations for precise and robust qPCR efficiency assessments. Biomol Detect Quantif 3:9-16. https://doi.org/10.1016/j.bdq.2015.01.005.
Tofilski A (2009) Shorter-lived workers start foraging earlier. Insect Soc 56:359-366. https://doi.org/10.1007/s00040-009-0031-3.
Varnon CA, Dinges CW, Black TE, Wells H, Abramson CI (2018) Failure to find ethanol-induced conditioned taste aversion in honey bees (Apis mellifera L.). Alcohol Clinic Exp Res 42:1260-1270. https://doi.org/10.1111/acer.13761.
de Vega C, Herrera CM, Johnson SD (2009) Yeasts in floral nectar of some South African plants: quantification and associations with pollinator type and sugar concentration. S Afr J Bot 75:798-806. https://doi.org/10.1016/j.sajb.2009.07.016.
Warrington JA, Nair A, Mahadevappa M, Tsyganskaya M (2000) Comparison of human adult and fetal expression and identification of 535 housekeeping/maintenance genes. Physiol Genomics 2:143-147. https://doi.org/10.1152/physiolgenomics.2000.2.3.143.
Wegener J, Huang ZY, Lorenz MW, Bienefeld K (2009) Regulation of hypopharyngeal gland activity and oogenesis in honeybee (Apis mellifera) workers. J Insect Physiol 55:716-725. https://doi.org/10.1016/j.jinsphys.2009.05.003.
Wiens F, Zitzmann A, Lachance M-A, Yegles M, Pragst F, Wurst FM, von Holst D, Guan SL, Spanagel R (2008) Chronic intake of fermented floral nectar by wild treeshrews. PNAS 105:10426-10431. https://doi.org/10.1073/pnas.0801628105.
Winston ML (1987) The biology of the honey bee. Harvard Univ Press, USA. ISBN: 9780674074095.
Wolf FW, Heberlein U (2003) Invertebrate models of drug abuse. J Neurobiol 54:161-178. https://doi.org/10.1002/neu.10166.
Woyciechowski M, Kozłowski J (1998) Division of labor by division of risk according to worker life expectancy in the honey bee (Apis mellifera L.). Apidologie 29:191-205. https://doi.org/10.1051/apido:19980111.
Wright GA, Lillvis JL, Bray HJ, Mustard JA (2012) Physiological state influences the social interactions of two honeybee nest mates. PLOS ONE 7:e32677. https://doi.org/10.1371/journal.pone.0032677.
Acknowledgements
We thank Brian Bladgen (Scottish Environment Protection Agency, UK) for proper English editing and the two anonymous reviewers for constructive comments.
Author information
Authors and Affiliations
Contributions
KM conceived this research and designed experiments; DS participated in the design and interpretation of the data; KM, DS, AK, LP and KK performed experiments and analysis; KM wrote the paper and participated in the revisions of it. All authors read and approved the final manuscript.
Corresponding author
Additional information
Manuscript editor: Yves Le Conte
La résistance aux intoxications alcooliques et les niveaux d'alcool déshydrogénase diffèrent selon les castes d'abeilles.
alcoolisme / éthanol / abeille mellifère / intoxication / effet de tolerance.
Resistenz gegen Alkoholvergiftung und Alkoholdehydrogenase-Mengen der Kasten der Honigbiene.
Alkoholismus / Ethanol / Honigbiene / Vegiftung / Toleranzeffekt.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Figure 1.
Representative PCR products digitalised after gel electrophoresis, and relative quantification (RQ) of Drosophila ADH as well as ADH1 in the three worker castes (N, R & F stand for nurses, reverted nurses and foragers, respectively). Stars indicate statistical significance with p < 0.001 as tested by the Dunnett test. (TIF 10011 kb)
Supplementary Figure 2.
Representative images of the Western blot (whole filter and stained gel picture). (TIF 1746 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Miler, K., Stec, D., Kamińska, A. et al. Alcohol intoxication resistance and alcohol dehydrogenase levels differ between the honeybee castes. Apidologie 52, 230–241 (2021). https://doi.org/10.1007/s13592-020-00812-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13592-020-00812-y