Abstract
Supplementation of the spent oyster substrate enhances its nutritional properties to produce a new mushroom cropping cycle. The study investigated the potential of a nano-fertilizer (Lithovit®-Amino25) with an admixture of 25% l-amino acids on Pleurotus ostreatus production, proteins, and amino acid contents. The product applied at spawning (t1), after the first harvest (t2), and at both timings (t3), in two doses: 3 g/kg (C1) or 5 g/kg (C2). Compared with control (C0t0), the first harvest was earlier by 2.3–3.3 days in C1t1 and C2t1. The biological yield of the second harvest was improved by 28.0% in C2t2. Superior results were in C1t3 where the number of crops increased to four, biological efficiency was optimized (117.3%) at the third harvest, and biological and economic yields increased by 36.7% and 36.4%, respectively. Lignin was the most degraded in C1t3, while residual cellulose, hemicellulose, neutral detergent fiber, and acid detergent fiber were higher in all treated substrates than in control. In C2t1, mushrooms were the richest in proteins, while in C1t1, they were the richest in the essential amino acids threonine, valine, isoleucine, leucine, and histidine. Lithovit®-Amino25 has a high potential for use in P. ostreatus production.
Similar content being viewed by others
Introduction
In the last decade, the acknowledgment that conventional farming technologies would not have the option to build profitability any further has increased nanotechnology’s interest (Mukhopadhyay 2014). Development in agriculture can be accomplished uniquely by increasing productivity through nanotechnology’s effective use (Selva and Balakrishnan 2017). Several research studies have pointed out their effects on improving growth, yield, and quality parameters of crops (Duhan et al. 2017), like tomato (Sajyan et al. 2018, 2019), grapevines (Sabir et al. 2014; Sassine et al. 2019) and many others. By concentrating on the exceptional properties of materials rising out of nanometric size, nanotechnology has the potential to revolutionize in the food sector (Baruah et al. 2008).
Mushrooms are edible fungi of commercial importance (Shivhare et al. 2004), used for therapeutic and nourishment purposes (Wani et al. 2010). Mushrooms of Pleurotus species, commonly known as oyster mushrooms, are globally highly intriguing for production because of their capacity to develop in a wide range of temperatures and use accessible lignocellulosic materials (Stamets 2000; Baysal et al. 2003; Royse 2003). In particular, Pleurotus ostreatus (Jacq.). P. Kumm. 1871 is the second-largest commercially cultivated edible mushroom, constituting approximately 27% of the total global production (Royse 2014). On an industrial scale, P. ostreatus is grown on cereal straw, mainly wheat straw (Rühl and Kües 2007). However, in many regions of the world, wheat straw is becoming less available and expensive (Masevhe et al. 2015; Picornell-Buendía et al. 2016a).
In mushroom producing regions, the spent mushroom substrate (SMS), which is the growing material left after several mushroom harvests, is generated in large amounts as 1 kg of fresh mushrooms brings about 5 kg of a spent substrate (i.e., 2 kg dry weight) (Finney et al. 2009). SMS are bulky products long considered a waste stream (Pardo-Giménez et al. 2012). The traditional methods of discarding or burning it are neither eco-friendly nor economic (Oei et al. 2007; Carrasco et al. 2018). On the other hand, SMS is highly nutritious as it is composed of lignocellulosic residues and fungal mycelium. Thus, it constitutes an accessible and low-cost substrate for mushroom cultivation (Grimm and Wösten 2018). However, SMS may not produce excellent mushroom yield by itself because of the reduction in nutrients due to their subsequent utilization by mushroom mycelium (Sharma and Jandaik 1985). Recycling of such substrate through amendment with nutritional supplements, especially protein-rich ones, to help further mushroom production is a practical choice to adapt to the high volume of this waste material (Pardo-Giménez et al. 2011; Pardo-Giménez et al. 2012; Picornell-Buendía et al. 2015, 2016a, b).
Commercial nutritional supplements initially developed for use in the cultivation of Agaricus spp. are based on proteins, lipid/protein blends, carboxylic acids, or minerals (Burton et al. 2015). On an experimental scale, amino acids could ameliorate mushroom performance (Sanchez et al. 2002). Still, their use as nutritional additives for P. ostreatus is tested only in submerged liquid cultures (Adebayo-Tayo et al. 2011). On a general basis, during mushroom cultivation, the supplement’s choice, and the correct timing and application methods are fundamental for getting the expected outcomes (Desrumaux et al. 1999). Usually, supplements with slow nutrient release formulas are applied at the end of the substrate preparation, to promote vegetative development all through the substrate (Naraian et al. 2009). They are also used at the end of the spawn run to advance the mushroom colonization and improve mushroom fructification (Pardo-Giménez et al. 2016).
Furthermore, nanometric size supplements, specially developed for mushroom cultivation, have not been reported yet. Otherwise, the use of nano-fertilizers, initially developed for use on plant crops as supplements for the growing substrate of P. ostreatus, had recently come out with meaningful results, mainly when the product was applied twice during the production cycle (Naim et al. 2020). Consequently, the current study investigated the effect of a nano-fertilizer containing an admixture of 25% l-amino acids, in different doses and application timings on P. ostreatus growth, production, and amino acid composition.
Materials and methods
Experimental treatments
The effect of Lithovit®-Amino25 (assigned as nano-amino), sourced from Tribodyn AG Company, Northelm, Germany, was tested in two separate doses: C1: 3 g/kg, C2: 5 g/kg, and three timings of application: t1: at spawning, t2: after the first harvest, t3: at spawning and after the first harvest. Six experimental treatments: C1t1, C1t2, C2t1, C2t2, C1t3, and C2t3, were arranged in a complete factorial design with two factors (dose and timing of application) and ten replicates (bags) per treatment. Experimental treatments were compared to a non-treated substrate or control (C0t0). Lithovit®-Amino25 is a nitrogen fertilizer with a 25% admixture of 16 water-soluble vegetable l-amino acids, suitable for use in organic farming (according to Regulation (EC) No. 834/2007-European Community), and having the following composition: 50.0% calcium carbonate (CaCO3), 28.0% calcium oxide (CaO), 9.0% silicon dioxide (SiO2), 3.0% total nitrogen (N), 1.8% magnesium oxide (MgO), 0.5% iron (Fe), and 0.02% manganese (Mn). The product is obtained from the addition of highly energized 16 water-soluble l-Amino acids to Lithovit particles, created by tribodynamic activation and micronization of dolomite (Bilal 2010).
Substrate preparation, spawning and cropping
The spent substrate was procured by a local mushroom farm “Gourmet” after one growing oyster mushroom cycle. After 1 week of sun-drying, the substrate shopped and mixed with wheat straw (1:1, w/w mixture). The resulting mixture was pasteurized using boiling water for 8 h at 60–65 °C and then left to cool down to 25 °C (spawning temperature) (Pardo-Giménez et al. 2012). After that, spawning was done using grain spawn of M2175 strain, imported from Mycelia Company, Deinze, Belgium, at a 5% rate (50 g of spawn per kg of the substrate). Spawned substrates were filled into perforated transparent polyethylene bags of 60 × 40 cm, with holes of 20 mm diameter at their sides. Bags were then incubated in a cropping chamber in dark conditions at 23–25 °C. The room was well-sealed, with climate control facilities. It was continuously moistened to keep relative humidity levels in the range of 80–90%. At the end of spawn run stage (complete substrate colonization), stimulation of fruit body formation was carried out by lighting (200 LUX light source), reduction of the temperature inside the chamber to around 15 °C, and ventilation to lower CO2 levels and maintain them below 700 ppm.
Chemical analysis
At the Lebanese Agricultural Research Institute (LARI)-Tal Amara station, samples of the initial substrate (Table 1) were evaluated for moisture (%), organic matter (%), carbon (%), and nitrogen (%) contents, as well as C/N ratio, and pH. Total carbohydrates (%) (Anthrone method), total protein (%) (Kjeldahl method), crude fiber (%) (AOAC 962.09 standards), and total fat content (AOAC 1984) were evaluated at the “Lebanese Food Drugs and Chemicals Administration (LFDCA)” Lebanese University-Hadath. Furthermore, fiber fractions of residual substrates; cellulose, hemicellulose, lignin, neutral detergent fiber (NDF), acid detergent fiber (ADF), and acid detergent lignin (ADL) were determined on dry samples by ANKOM technology method, filter bag technique (08-16-06, 08 − 05) following AOAC official method of analysis (AOAC 2019a, b). Moreover, the mushroom composition was analyzed using samples of the mushroom pileus. Total protein content was determined using the macro-Kjeldahl method (N × 4.38) (Reis et al. 2012). Analysis of amino acid composition was performed using the Young Li amino acid analyzer applying fluorescence detection using o-phthalaldehyde (OPA) and post-column derivatization method. The amino acid analysis was performed using native samples of mushrooms which were sampled at first (concerning the treatments C1t1 and C2t1), second (concerning the treatments C1t2 and C2t2), or third (concerning the treatments C1t3 and C2t3) harvest. The different tests were performed in triplicates.
Measurements
The date of spawn run initiation (days after spawning: DAS) indicated the appearance of first white mycelial patches at the inner side of inoculated bags. Before filling the bags with inoculated substrates, squares of 5 × 5 cm were drawn on their sides. The time to half and complete mycelial colonization of the substrate was recorded when half or all squares became white. The surface mycelial density corresponded to the degree of substrate colonization by the mycelium. It was evaluated at the time of complete mycelial colonization by assigning: (1) to poor running growth, (2) to mycelium growing throughout the bag but not uniformly white, and (3) to mycelium growing throughout the bag and uniformly white (Yang et al. 2013). The number of mushroom bunches, the weight of bunches, fruit body number, and fruit body weight were recorded at each harvest. Economic yield corresponded to mushrooms’ total weight after removal of the base of stalks (Girmay et al. 2016). Biological efficiency (BE) was determined per treatment as: total fresh weight of mushrooms (g)/initial dry weight of substrate (g) × 100 (Oseni et al. 2012). At each harvest, ten representative mushrooms of uniform size were sampled from each treatment to evaluate the pileus diameter and length and stipe diameter and length, using a sliding caliper.
Data analysis
Statistical analysis was carried out using SPSS 25®, by applying one-way ANOVA and Duncan’s multiple range tests. Pearson’s correlations and stepwise multiple regression analysis were applied to investigate the relation between the biological yield and mushroom indicators, and test the contribution of each mushroom indicator (as a predictor) to variation of economic yield at each harvest. All tests applied considering a Pvalue < 0.05.
Results
Mycelia growth and first harvest
Assessment of mycelia growth (dates of spawn run initiation, 50% substrate colonization, 100% substrate colonization, and mycelial density), and pinhead initiation (Table 2) proved a non-significant effect of supplementation compared with control. However, the first harvest was earlier by 3.3 and 2.3 d following the application of the respective doses 3 g/kg and 5 g/kg of nano-amino at spawning.
Mushroom production at the first harvest
Supplementation of the growing substrate with nano-amino at spawning has caused a significant reduction in the average fruit body number, bunches weight, and economic yield at the first harvest (Table 3). On the contrary, it caused a significant improvement in the average fruit body weight (by 18.1 and 12.4 g on average), pileus diameter (by 4.5 and 3.4 cm on average), pileus length (by 2.0 and 2.7 cm on average), and the ratio PD/SL (by 0.3 and 0.5) with the respective doses of 3 g/kg and 5 g/kg. The average stipe diameter increased by 0.3 cm with 5 g/kg nano-amino, and the average stipe length by 2 cm with 3 g/kg nano-amino. The first harvest’s biological and economic yields decreased significantly in the treatment C2t1 compared to control (reduction by 22.7% and 21.0% on average).
Mushroom production at the second harvest
In comparison with control, the average number of bunches at the second harvest (Table 4) increased by 2.0 following 3 g/kg nano-amino application at timing 2 (C1t2). Additionally, the average fruit body number was higher by 11.3 and 6.3 following 5 g/kg nano-amino application at the respective timings 2 and 3. Besides, a significant improvement was recorded in the bunches’ weight of C2t2 (by 58.5 g on average), and fruit body weight (by 10.5 g on average) of C1t3. Pileus diameter and pileus length increased in the treatments C1t1 (by 1.8 and 0.9 cm on average) and C1t3 (by 2.2 and 0.8 cm). On the other hand, both tested doses applied at timing 2 caused a significant average length reduction. Also, 3 g/kg nano-amino application at timing 2 has shortened the average stipe length by 1.4 cm, resulting in a significantly higher PD/SL ratio than control. In the second harvest, biological and economic yield increased by 22.0% and 25.6% in C1t1, 28.0% and 25.1% in C2t2, and 25.8% and 25.9% in C2t3.
Mushroom production at the third harvest
Supplementation of the growing substrate at spawning with 3 g/kg nano-amino caused a significant increase in bunches number (by 3.3 on average), and fruit body number (by 11.7 on average), coupled with a significant reduction in bunches weight (by 25.6 g on average), and fruit body weight (by 26.1 g on average) at the third harvest compared with control (Table 5). Otherwise, when the same product dose was applied twice (treatment C1t3), it improved bunches weight (by 82.4 g on average) and fruit body weight (by 45.5 g on average). In all treatments, the pileus diameter and stipe diameter were significantly lower at the third harvest compared to control. A similar trend was observed for stipe length (except in C1t3), pileus length (except in C1t3), and PD/SL ratio (except in C2t3). Production at the third harvest was comparable to control, except in C1t3, where biological and economic yields increased by around 36.7% and 36.4%.
Total production per treatment
The harvest’s number (Table 6) rose from 3.0 in control to 4.0 in C1t3 and decreased to 2.0 in C1t2. Additionally; the double application of 3 g/kg nano-amino improved the total biological and economic yields by around 23.2 and 25.3%, respectively, and resulted in the highest biological efficiency (117.3%).
Variation of biological yield per harvest and in total
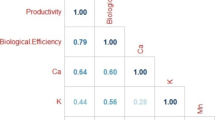
Results in Table 7 demonstrated that the total biological yield was strongly positively correlated with biological yields of the first (R = 0.51, P = 0.02) and third (R = 0.91, P = 0.00) harvests, but was the most strongly correlated with the latter. The most significant model (model1, R2 = 0.84) resulting from stepwise regression (Table 8) showed that the biological yield of the third harvest had a higher positive contribution (highest positive coefficient) to variation in total biological yield compared to that obtained at the first harvest.
The biological yield of the first harvest was positively correlated with the fruit body number (R = 0.95, P = 0.00), and negatively correlated with stipe diameter (R = – 0.83, P = 0.00) and pileus length (R = – 0.69, P = 0.02), obtained at the same harvest. The most significant model resulting from stepwise regression (model 3, R2 = 0.98) showed that the stipe diameter was the predictor contributing the most (highest coefficient) to variation in the first harvest’s biological yield. Moreover, there was a strong positive correlation between the biological yield and fruit body number (R = 0.72, P = 0.00), and bunches weight (R = 0.51, P = 0.01) of the second harvest. The fruit body number was a good predictor of the variation in biological yield of the second harvest (positive coefficient, model 4); however, the most significant model of stepwise regression (model 5, R2 = 0.80) depicted a stronger contribution of the pileus diameter (higher coefficient). Biological yield of the third harvest was strongly and positively correlated with bunches weight (R = 0.72, P = 0.00), fruit body weight (R = 0.75, P = 0.00) and stipe length (R = 0.64, P = 0.00). Besides, fruit body weight, bunches number, and stipe diameter were good predictors to variation of the biological yield of the third harvest. However, the stipe diameter had the highest contribution (highest coefficient, model 8). Noteworthy is that stipe diameter had a negative contribution to biological yields of the first and third harvests.
Fiber fractions in the residual substrate
Analysis of the spent substrate generated from each treatment (Table 9) showed that residual cellulose, hemicellulose, NDF, and ADF were the lowest in the control substrate than substrates initially treated by nano-amino. Concerning the latter, residual cellulose was significantly reduced in C2t3 compared to the remaining substrates (reduction by 9.4 units compared to the initial substrate). It was also more significantly reduced in C2t1 and C2t3 than in C1t1 and C1t3, and in C1t2 than in C2t2. Hemicellulose was more degraded with 5 g/kg than 3 g/kg nano-amino at all tested timings (reduction range of 8.5–11.6 units compared to 0.1–6.4 units, respectively). As a result, residual NDF was higher in the later substrates. Residual ADF was significantly lower in substrates initially treated with 5 g/kg at spawning than 3 g/kg. However, opposite results were obtained when the same respective doses were initially applied after the first harvest. Lignin was the more degraded when initial substrates were treated by the lower dose compared to the highest (reduction range of 3.03–5.81 units compared to 1.40–1.69, respectively), and was the most pronounced in C1t3 substrates. In the same substrates, residual ADL was the most reduced in comparison with the control.
Protein and amino acid content in mushrooms
Mushroom protein content (Table 10) decreased due to the nano-amino application compared to control, except in C2t1. There was an in some essential amino acids in C1t1 (threonine, valine, isoleucine, leucine, and histidine) and C2t3 (Threonine, histidine, and methionine) mushrooms. Also, nano-amino treatment had a significant effect on histidine content, which was improved in all treatments compared with control. On the contrary, it caused a decrease in the mushroom’s arginine content. Alanine content was higher than control, except in C1t3 and C2t3. Glutamic acid increased by 0.48, 0.28, 0.20, and 1.13 units in C1t1, C2t2, C1t3, and C2t3, respectively. Proline content was higher by 0.16 and 0.14 units in C1t1 and C2t3 than in control.
Discussion
Oyster mushroom develops well and gives best yield at pH slightly basic in nature (Khan et al. 2013). The application of a low dose of nano-amino, with high amounts of CaCO3 and CaO, from the spawning time, may have affected the initially low substrate pH (5.2) at the early growth stages of mycelium, thus the harvest date and the biological efficiency in treated substrates. However, the product’s effect on the substrate pH, consequently, on the growth and production of the mushroom requires further investigation in future studies to prove the above-stated assumptions.
Although biological yield was reduced at the first harvest, mostly due to reduced fruit body numbers, heavier mushrooms were obtained with a longer and a thicker stipe. Supplementation at spawning also had a delayed positive effect on the number of bunches and fruit bodies produced at the third harvest.
After testing the effect of supplementation after the first harvest, it seemed that a high product dose was essential to influence substrate productivity directly. In point of fact, treating the growing substrate with 5 g/kg at this timing has optimized the biological yield obtained at the second harvest (improvement of 28.0% compared to control). On the contrary, the application of 3 g/kg at this timing has limited the production to only two flushes. These findings suggest the slow release of nutrients by the tested product into the growing substrate. Due to their extensive surface area, nanoparticles can hold an abundance of nutrients and release it slowly and steadily facilitate their uptake (Selva and Balakrishnan 2017). Applying the product twice with 3 g/kg was the most effective; not only had it increased the number of harvests to four, but it optimized as well the biological efficiency (117.3%) from the first three harvests. A biological efficiency between 50 and 63% was obtained using wheat straw and spent Pleurotus substrate as a base material supplemented with a 120 g/6 kg dose of a protein-rich additive Calprozime® (Picornell-Buendía et al. 2015). Different tests to investigate the practicality of reusing such substrate in new production cycles had found, because of supplementation, increments of biological efficiency somewhere in the range of 51 and 70% (Zied et al. 2011). Nitrogen can be transported into the fungus’s living cell in the form of amino acids (Mikeš et al. 1994). The product applied was rich in amino acids, essential during the mycelial maturation stage (Du et al. 2019). When added to the growing substrate, amino acids, as source of nutrients, could be more easily assimilated than proteins present in the initial substrate. Mycelia development was ameliorated due to the addition of the amino acids glycine and leucine to the growing culture medium (Adebayo-Tayo et al. 2011).
Pleurotus ostreatus can decompose the cellular wall components present in the raw lignocellulosic material, like cellulose, lignin, and hemicellulose through the action of complex oxidative and hydrolytic enzymatic systems (Castro 2003; Fernández-Fueyo et al. 2016). Among others, hemicellulases, cellulases, and ligninases enzymes degrade long and insoluble parts of lignocellulosic materials into soluble components of low molecular weight that are taken by intracellular enzymes of fungi for their nutrition (Kurt and Buyukalaca 2010; Picornell-Buendía et al. 2015). Hemicellulose, cellulose, and lignin serve as an energy source for fungal growth because they contain carbon, hydrogen, and oxygen, clarifying their decrease along the cultivation cycle (Andrade et al. 2010). Lignin probably acts as a barrier to prohibit the mushroom from attacking polysaccharides. Therefore, access to holocellulose, the carbon and the energy source for this species, is enabled after lignin degradation (Xiao et al. 2017). P. ostreatus secretes Manganese peroxidase (MnP), one major oxidative enzyme responsible for lignin oxidation (Wan and Li 2012). Therefore, the highest lignin degradation obtained following the double application of 3 g/kg of Lithovit®-Amino25 containing manganese may be associated with an improved P. ostreatus MnP activity. Improvement in lignin degradation may be the cause behind the highest biological efficiency obtained in such treatment. This assumption may be confirmed by further investigating the extracellular enzymes’ secretion by a nano-amino supplemented mycelium.
In general, the abundance of nano-amino in the growing substrate significantly affected the pileus length of harvested mushrooms. In the treatment C1t3 the weight of fruit bodies increased at the third harvest, mainly because of larger pileus (length and diameter). Picornell-Buendía et al. (2015) obtained fruiting bodies of excellent weight on spent mushroom substrate supplemented with protein additives. Also, the mushroom shape was more uniform among consecutive harvests (almost similar PD/SL ratio at the second and third harvests). Still, mushrooms produced in such treatment at the second harvest were more marketable than those obtained in the non-treated substrate (higher PD/SL ratio). Mushrooms having large pileus and short stipes are more acceptable at the market (Synytsya et al. 2008).
The mushroom nutrient composition is affected by the substrate composition and properties (El Sebaaly et al. 2018, 2019; Abou Fayssal et al. 2020). The mushroom nutritional value may be improved as by applying nano-amino at spawning; proteins increase with the highest dose used, and essential amino acids with the lowest one. A similar improvement in mushroom protein content was reported on substrates based on wheat straw and SOS, enriched by protein-rich additives, wheat bran, or commercial additives (Picornell-Buendía et al. 2016a, b).
Pieces of evidence were provided by the present study that Lithovit®-Amino25 can be used in P. ostreatus cultivation, especially with 3 g/kg applied twice during the production cycle. In addition to the improvement in biological yield, farmers may optimize their benefits from P. ostreatus by saving in the initial substrate cost and the cost of supplements, since lower product doses are required to obtain good results.
Availability of data and materials
Authors apologize for not being able to share the data because it belongs to a PhD study that is not yet defended.
Abbreviations
- C:
-
Concentration
- t:
-
Timing
- NDF:
-
Neutral detergent fiber
- ADF:
-
Acid detergent fiber
- P. ostreatus :
-
Pleurotus ostreatus
- SMS:
-
Spent mushroom substrate
- CaCO3 :
-
Calcium carbonate
- CaO:
-
Calcium oxide
- SiO2 :
-
Silicon dioxide
- N:
-
Nitrogen
- MgO:
-
Magnesium oxide
- Fe:
-
Iron
- Mn:
-
Manganese
- L:
-
Lithovit
- SOS:
-
Spent oyster substrate
- C/N:
-
Carbon/nitrogen ratio
- AOAC:
-
Association of Official Analytical Chemists
- LFDCA:
-
Lebanese Food Drugs and Chemicals Administration
- ADL:
-
Acid detergent lignin
- OPA:
-
o-Phthalaldehyde
- DAS:
-
Days after spawning
- BE:
-
Biological efficiency
- SPSS:
-
Statistical Package for Social Sciences
- ANOVA:
-
Analysis of variance
- PD/SL:
-
Pileus diameter/stipe length ratio
- BY:
-
Biological yield
- BN:
-
Bunches number
- FBN:
-
Fruit body number
- FBW:
-
Fruit body weight
- PD:
-
Pileus diameter
- SD:
-
Stipe diameter
- SL:
-
Stipe length
- PL:
-
Pileus length
- BYT:
-
Total biological yield
- H:
-
Harvest
References
Abou Fayssal S, Alsanad MA, Sebaaly E, Ismail Z, Sassine AIH, YN (2020) Valorization of olive pruning residues through bioconversion into edible mushroom Pleurotus ostreatus (Jacq. Ex Fr.) P. Kumm. (1871) of improved nutritional value. Scientifica. 2020. https://doi.org/10.1155/2020/3950357
Adebayo-Tayo BC, Jonathan SG, Popoola OO, Egbomuche RC (2011) Optimization of growth conditions for mycelial yield and exopolysaccharride production by Pleurotus ostreatus cultivated in Nigeria. Afr J Microbiol Res 5:2130–2138
Andrade MCN, Zied DC, Minhoni MTA, Sansigolo CA (2010) Análise química da madeira e casca de diferentes tipos de eucalipto antes e durante o cultivo de shiitake em toras. Árvore 34:165–175
AOAC Official methods of Analysis (1984) 14th edn. Arlington, TX, USA
AOAC Official method of Analysis (2019a) 21th ed. No. 973.15 for (NDF) Chap. 4
AOAC Official method of Analysis (2019b) 21th ed. ADF and ADL method no. 2002.0 Chap. 4
Baruah S, Warad HC, Chindaduang A, Tumcharern G, Dutta J (2008) Studies on chitosan stabilised Zns: Mn2+ nanoparticles. Bionanoscience 2:42–48. https://doi.org/10.1166/jbns.2008.025
Baysal E, Peker H, Yalinkilic MK, Temiz A (2003) Cultivation of oyster mushroom on waste paper with some added supplementary materials. Bioresour Technol 89:95–97. https://doi.org/10.1016/S0960-8524(03)00028-2
Bilal BA (2010) Lithovit®: An innovative fertilizer. The 3rd e-conference on agricultural biosciences (IeCAB 2010), 1st–15th June 2010. http://www.slideserve.com/madison/lithovitan-innovative-fertilizer
Burton K, Noble R, Rogers S, Wilson J (2015) Understanding mushroom nutrition: project aimed at improving yield, substrate efficiency and utilization and favor. M056 Final Report: Agriculture and Horticulture Development board (AHDB)
Carrasco J, Zied DC, Pardo JE, Preston GM, Giménez AP (2018) Supplementation in mushroom crops and its impact on yield and quality. AMB Expr 8:146–156
Castro ALA (2003) Resíduo de lixadeira do algodão: Produção de cogumelo, ensilagem e alterações da composição bromatológica e degradabilidade. Diss., MSc. Universidade Federal de Lavras, Minas Gerais
Desrumaux B, Seydeyn P, Werbrouck A, Lannoy P (1999) Supplémenter dans la culture du champignon de couche: expérience comparative avec quelques produits de supplémentation du commerce. Bull FNSACC 81:789–802
Du F, Zou Y, Hu Q, Jing Y, Yang X (2019) Metabolic profiling of Pleurotus tuoliensis during mycelium physiological maturation and exploration on a potential indicator of mycelial maturation. Front Microbiol 9:3274. https://doi.org/10.3389/fmicb.2018.03274
Duhan JS, Kumar R, Duhan S (2017) Nanotechnology: the new perspective in precision agriculture. Biotechnol Rep 15:11–23
El Sebaaly Z, Abou Fayssal S, Shaban N, Sassine YN (2018) Growing Agaricus bisporus on compost mixtures based on chicken manure and banana residues. In: Proceedings of the 9th international scientific agriculture symposium agrosym, Jahorina, Bosnia and Herzegovina, December, pp 1172–1180
El Sebaaly Z, Assadi F, Sassine YN, Shaban N (2019) Substrate types effect on the nutritional composition of button mushroom (Agaricus bisporus). Agric For 65(1):73–80. https://doi.org/10.17707/AgricultForest.65.1.08
Fernández-Fueyo E, Ruiz-Dueñas FJ, López-Lucendo MF, Pérez-Boada M, Rencoret J, Gutiérrez A, Pisabarro AG, Ramírez L, Martínez AT (2016) A secretomic view of woody and nonwoody lignocellulose degradation by Pleurotus ostreatus. Biotechnol Biofuels 9:1–18. https://doi.org/10.1186/s13068-016-0462-9
Finney KN, Ryu C, Sharifi VN, Swithenbank J (2009) The reuse of spent mushroom compost and coal tailings for energy recovery: comparison of thermal treatment technologies. Bioresour Technol 100:310–315.https://doi.org/10.1016/j.biortech.2008.05.054
Girmay Z, Gorems W, Birhanu G, Zewdie S (2016) Growth and yield performance of Pleurotus ostreatus (Jacq. Fr.) Kumm (oyster mushroom) on different substrates. AMB Expr 6:1–7. https://doi.org/10.1186/s13568-016-0265-1
Grimm D, Wösten HAB (2018) Mushroom cultivation in the circular economy. Appl Microbiol Biotechnol 102:7795–7803. https://doi.org/10.1007/s00253-018-9226-8
Khan MW, Ali MA, Khan NA, Khan MA, Javed AR, Javed N (2013) Effect of different levels of lime and pH on mycelial growth and production efficiency of oyster mushroom (Pleurotus spp.). Pak J Bot 45:297–302
Kurt S, Buyukalaca S (2010) Yield performances and changes in enzyme activities of Pleurotus spp. (P. ostreatus and P. sajor-caju) cultivated on different agricultural wastes. Bioresour Technol 101:3164–3169. https://doi.org/10.1016/j.biortech.2009.12.011
Masevhe MR, Soundy P, Taylor NJ (2015) Alternative substrates for cultivating oyster mushrooms (Pleurotus ostreatus). S Afr J Plant Soil 33:1–8. https://doi.org/10.1080/02571862.2015.1079932
Mikeš V, Zofall M, Chytil M, Fulneček J, Scháně L (1994) Ammonia-assimilating enzymes in the basidiomycete fungus Pleurotus ostreatus. Microbiology 140:977–982. https://doi.org/10.1099/00221287-140-4-977
Mukhopadhyay SS (2014) Nanotechnology in agriculture: prospects and constraints. Nanotechnol Sci Appl 7:63–71. https://doi.org/10.2147/NSA.S39409
Naim L, Alsanad MA, Sebaaly E, Shaban Z, Abou Fayssal N, Sassine S, YN (2020) Variation of Pleurotus ostreatus (Jacq. Ex Fr.) P. Kumm. (1871) performance subjected to different doses and timings of nano-urea. Saudi J Biol Sci 27:1573–1579
Naraian R, Sahu R, Kumar S, Garg S, Singh C, Kanaujia R (2009) Influence of different nitrogen rich supplements during cultivation of Pleurotus florida on corn cob substrate. Environmentalist 29:1
Oei P, Hui Z, Jianhua L, Jianqing D, Meiyuan C, Yi C (2007) The alternative uses of spent mushroom compost. In: Proceedings of the 6th international conference on mushroom biology and mushroom products, Bonn, Germany, 29 September–3 October, pp 231–245
Oseni O, Dube S, Wahome P, Masarirambi M, Earnshaw DM (2012) Effect of wheat bran supplement on growth and yield of oyster mushroom (Pleurotus ostreatus) on fermented pine sawdust substrate. Exp Agric Hortic 12:30–40
Pardo-Giménez A, Pardo-González JE, Zied DC (2011) Evaluation of harvested mushrooms and viability of Agaricus bisporus growth using casing materials made from spent mushroom substrate. Int J Food Sci Technol 46:787–792. https://doi.org/10.1111/j.1365-2621.2011.02551.x
Pardo-Giménez A, Picornell Buendia MR, de Juan Valero JA, Pardo-Gonzalez JE, Zied CD (2012) Cultivation of Pleurotus ostreatus using supplemented spent oyster mushroom substrate. Acta Hortic 933:267–272. https://doi.org/10.17660/ActaHortic.2012.933.33
Pardo-Giménez A, Catalán L, Carrasco J, Álvarez-Ortí M, Zied D (2016) Effect of supplementing crop substrate with defatted pistachio meal on Agaricus bisporus and Pleurotus ostreatus production. J Sci Food Agric 96:838–3845. https://doi.org/10.1002/jsfa.7579
Picornell-Buendía MR, Pardo A, de Juan JA (2015) Reuse of degraded Pleurotus ostreatus substrate through supplementation with wheat bran and Calprozime® quantitative parameters. Agron Colomb 33:261–270. https://doi.org/10.15446/agron.colomb.v33n2.49760
Picornell-Buendía MR, Pardo-Giménez A, Juan-Valero D, Arturo J (2016a) Agronomic qualitative viability of spent Pleurotus substrate and its mixture with wheat bran and a commercial supplement. J Food Qual 39:533–544. https://doi.org/10.1111/jfg.12216
Picornell-Buendía R, Pardo-Giménez A, de Juan-Valero JA (2016b) Agronomic assessment of spent substrates for mushroom cultivation. Biotechnol Agron Soc Environ 20:363–374
Reis FS, Barros L, Martins A, Ferreira ICFR (2012) Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms: an inter-species comparative study. Food Chem Toxicol 50:191–197. https://doi.org/10.1016/j.fct.2011.10.056
Royse DJ (2003) Cultivation of oyster mushrooms. College of Agricultural Sciences. Pennsylvania State University, Pennsylvania
Royse DJ (2014) A global perspective on the high five: Agaricus, Pleurotus, Lentinula, Auricularia and Flammulina. In: Proceedings of the 8th international conference on mushroom biology and mushroom products, New Delhi, pp 1–6
Rühl M, Kües U (2007) Mushroom production. In: Kües U (ed) Wood production, food technology, and biotechnological impacts. Universitatsverlag Gottingen, Gottingen, pp 555–586
Sabir A, Yazar K, Sabir F, Kara Z, Atilla M, Goksu N (2014) Vine growth, yield, berry quality attributes and leaf nutrient content of grapevines as influenced by seaweed extract (Ascophyllum Nodosum) and nanosize fertilizer pulverizations. Sci Hortic 175:1–8. https://doi.org/10.1016/j.scienta.2014.05.021
Sajyan TK, Shaban N, Rizkallah J, Sassine YN (2018) Effects of monopotassium phosphate, nano-calcium fertilizer, acetyl salicylic acid (Aspirin) and glycinebetaine application on growth and production of tomato (Solanum lycopersicum) crop under salt stress. Agron Res 16:872–883
Sajyan TK, Naim L, Sebaaly Z, Rizkallah J, Shaban N, Sassine YN (2019) Alleviating the adverse effects of salinity stress on tomato crop (Solanum lycopersicum) using nano-fertilizer as foliar application. In: XXX international horticultural congress IHC2018: international symposium on water and nutrient relations and management of horticultural crops. Istanbul, Turkey, August 12, pp 33–39. https://doi.org/10.17660/ActaHortic.2019.1253.5
Sanchez JE, Royse DJ, Hernandez G (2002) Development of non-composted substrate for production of Agaricus bisporus. In: Sanchez JE, Huerta G, Montiel E (eds) Proceedings of the 4th international conference on mushroom biology and mushroom products, Cuernavaca, Mexico, pp 265–270
Sassine YN, Al Turki SM, Sebaaly E, Bachour Z, Masri L, IY (2019) Finding alternatives for Dormex (Hydrogen Cyanamid) as dormancy breaking agent. Fresen Environ Bull 28:10214–10224
Selva PP, Balakrishnan N (2017) A review of nano fertilizers and their use and functions in soil. Int J Curr Microbiol Appl Sci 6:3117–3133
Sharma VP, Jandaik CL (1985) Studies on recycling of Pleurotus waste. Mushroom J Tropics 6:13–15
Shivhare U, Arora S, Ahmed J, Raghavan G (2004) Moisture adsorption isotherms for mushroom. Food Sci Technol-Leb 37:133–137. https://doi.org/10.1016/S0023-6438(03)00135-X
Stamets P (2000) Growing gourmet and medicinal mushrooms, 3 edn. Ten Speed Press, Berkeley
Synytsya A, Mícková K, Jablonsky I, Sluková M, Copíková J (2008) Mushrooms of genus Pleurotus as a source of dietary fibres and glucans for food supplements. Czech J Food Sci 26:441–446
Wan C, Li Y (2012) Fungal pretreatment of lignocellulosic biomass. Biotechnol Adv 30:1447–1457
Wani B, Bodha R, Wani H (2010) Nutritional and medicinal importance of mushrooms. J Med Plants Res 4:2598–2604. https://doi.org/10.5897/JMPR09.565
Xiao Q, Ma F, Li Y, Yu H, Li C, Zhang X (2017) Differential proteomic profiles of Pleurotus ostreatus in response to lignocellulosic components provide insights into divergent adaptive mechanisms. Front Microbiol 8:480. https://doi.org/10.3389/fmicb.2017.00480
Yang Z, Xu J, Fu Q, Fu X, Shu T, Bi Y, Song B (2013) Antitumor activity of a polysaccharide from Pleurotus eryngii on mice bearing renal cancer. Carbohydr Polym 95:615–620. https://doi.org/10.1016/j.carbpol.2013.03.024
Zied DC, Savoie JM, Pardo-Giménez A (2011) Soybean and nutrition, 1 edn. InTech Open Access, Rijeka
Acknowledgements
Authors want to recognize Tribodyn AG Company, Northelm, Germany for offering Lithovit®-Amino25.
Funding
The authors declare that they had not received external funds.
Author information
Authors and Affiliations
Contributions
This work was carried out in collaboration between all authors. Author LN carried out the field work and managed the literature search and wrote the draft of the manuscript. Author SAF carried out data analysis and processing. Authors ZES, MS and YNS corrected, edited and reviewed the manuscript. Authors YNS and NS supervised the whole work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Naim, L., Alsanad, M.A., Shaban, N. et al. Production and composition of Pleurotus ostreatus cultivated on Lithovit®-Amino25 supplemented spent substrate. AMB Expr 10, 188 (2020). https://doi.org/10.1186/s13568-020-01124-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13568-020-01124-1