Chitosan-Based Delivery Systems Loaded with Glibenclamide and Lipoic Acid: Formulation, Characterization, and Kinetic Release Studies
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Polymeric Systems
2.3. Characterization of the Polymeric Systems
2.3.1. Particle Size and Morphology
2.3.2. Fourier Transform Infrared Spectroscopy (FT-IR)
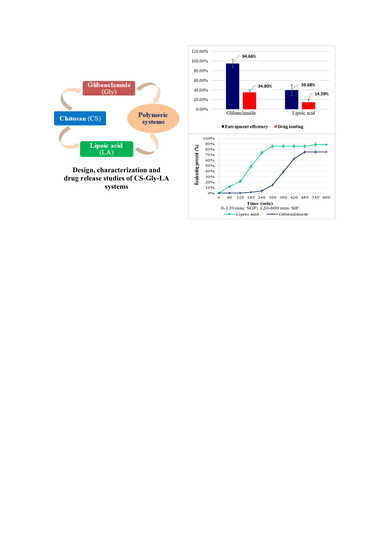
2.3.3. Yield, Entrapment Efficiency, and Drug Loading
- m1 = the amount of obtained microparticles, in dry state (mg)
- m0 = the total initial amount of CS and active substances (Gly, LA) (mg)
- m3 = the amount of active substance loaded into CS microparticles (mg)
- m0 = the initial amount of active substance used for the preparation of microparticles (mg)
- m3 = the amount of active substance loaded into chitosan microparticles (mg)
- m1 = the amount of microparticles obtained, in dry state (mg)
2.3.4. Swelling Degree
- w1 = the weight of the swollen microparticles at different timelines (mg)
- w2 = the weight of the dried microparticles (mg)
2.3.5. KineticDrug Release Studies
- m0 = the initial amount of active substance (Gly, LA) loaded into microparticles (mg)
- mt = the amount of active substance released, at a specific time, in simulated media (mg)
3. Results
3.1. Particle Size and Morphology
3.2. Fourier Transform Infrared Spectroscopy (FT-IR)
3.3. Yield, Entrapment Efficiency, and Drug Loading
3.4. Swelling Degree
3.5. Kinetic Release Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tan, G.D.; Kozlowska, O.; Rea, R.D. Delivery and organization of diabetes care: Integrated care. Medicine 2018, 47, 127–130. [Google Scholar] [CrossRef] [Green Version]
- Constantin, S.M.; Lupascu, F.G.; Apotrosoaei, M.; Focsa, A.V.; Vasincu, I.M.; Confederat, L.G.; Dimitriu, G.; Lupusoru, C.E.; Routier, S.; Buron, F.; et al. Antidiabetic effects and safety profile of chitosan delivery systems loaded with new xanthine-thiazolidine-4-one derivatives: Invivo studies. J. Drug Deliv. Sci. Technol. 2020, 60, 102091. [Google Scholar] [CrossRef]
- Barzkar, H.; Nikbakht, H.A.; Zeinolabedini, M.; Babazadeh, T.; Hassanipour, S.; Ghaffari, S. Factors associated with therapeutic target achievement in the control of complications in consequence of diabetes: A hospital-based study in west of Iran. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 2009–2013. [Google Scholar] [CrossRef] [PubMed]
- Selim, S. Frequency and pattern of chronic complications of diabetes and their association with glycemic control. Diabetes Metab. Syndr. Clin. Res.Rev. 2017, 11S, S311–S314. [Google Scholar] [CrossRef] [PubMed]
- Avogaro, A.; Fadini, G.P. Microvascular complications in diabetes: A growing concern for cardiologists. Int. J. Cardiol. 2019, 291, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Pickett, K.A. Microvascular Complications of Diabetes: What’s Relevant for Practice? J. Nurse Pract. 2016, 12, 683–690. [Google Scholar] [CrossRef]
- Dewi, F.; Hinchliffe, R.J. Foot complications in patients with diabetes. Surgery 2019, 37, 106–111. [Google Scholar] [CrossRef] [Green Version]
- Fisher, M. Macrovascular disease in diabetes. Medicine 2006, 34, 101–103. [Google Scholar] [CrossRef]
- Vella, S.; Petrie, J.R. Macrovasculardisease: Pathogenesis and risk assessment. Medicine 2015, 43, 1–6. [Google Scholar] [CrossRef]
- Lupascu, F.G.; Giusca, S.E.; Caruntu, I.D.; Anton, A.; Lupusoru, C.E.; Profire, L. The safety profile of new antidiabetic xanthine derivatives and their chitosan based formulations. Eur. J. Pharm. Sci. 2019, 127, 71–78. [Google Scholar] [CrossRef]
- Abbasa, G.; Al Harrasi, A.; Hussain, H.; Hamaed, A.; Supuran, C.T. The management of diabetes mellitus-imperative role of natural products against dipeptidyl peptidase-4, α-glucosidase and sodium-dependent glucose co-transporter 2 (SGLT2). Bioorg. Chem. 2019, 86, 305–315. [Google Scholar] [CrossRef]
- Padhi, S.; Nayak, K.A.; Behera, A. Type II diabetes mellitus: A review on recent drug based therapeutics. Biomed. Pharmacothr. 2020, 131, 110708. [Google Scholar] [CrossRef] [PubMed]
- Seino, S.; Takahashi, H.; Takahashi, T.; Shibasaki, T. Treating diabetes today: A matter of selectivity of sulphonylureas. Diabetes Obes. Metab. 2012, 14 (Suppl. 1), S9–S13. [Google Scholar] [CrossRef] [PubMed]
- Thule, P.M.; Umpierrez, G. Sulfonylureas: A new look at old therapy. Curr. Diab. Rep. 2014, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Avram, I.; Lupascu, F.G.; Confederat, L.; Constantin, S.M.; Stan, C.I.; Profire, L. Chitosan microparticles loaded with antidiabetic drugs—Preparation and characterization. Farmacia 2017, 65, 443–448. [Google Scholar]
- Leao, D.A.; Profiro, J.H.O.; Nunes, L.C.C.; Silva-Filho, E.C.; Soares, M.F.R.; Soares-Sobrinho, J.L. Strategies to improve glibenclamide dissolution: A review using database tomography. JDDST 2019, 54, 101242. [Google Scholar] [CrossRef]
- Ighodaro, O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 2018, 108, 656–662. [Google Scholar] [CrossRef]
- Ullah, A.; Khan, A.; Khan, I. Diabetes mellitus and oxidative stress—A concise review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar]
- Yuan, T.; Yang, T.; Chen, H.; Fu, D.; Hu, Y.; Wang, J.; Yuan, Q.; Yu, H.; Xu, W.; Xie, X. New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis. Redox Biol. 2019, 20, 247–260. [Google Scholar] [CrossRef]
- Rahimi-Madiseh, M.; Malekpour-Tehrani, A.; Bahmani, M.; Rafieian-Kopaei, M. The research and development on the antioxidants in prevention of diabetic complications. Asian Pac. J. Trop. Med. 2016, 9, 825–831. [Google Scholar] [CrossRef] [Green Version]
- Goraca, A.; Huk-Kolega, H.; Piechota, A.; Kleniewska, P.; Ciejka, E.; Skibska, B. Lipoic acid—Biological activity and therapeutic potential. Pharmacol. Rep. 2011, 63, 849–858. [Google Scholar] [CrossRef]
- Shay, K.P.; Moreau, R.; Smith, E.J.; Smith, A.R.; Hagen, T.M. Alpha-lipoic acid as a dietary supplement: Molecular mechanisms and therapeutic potential. Biochim. Biophys. Acta 2009, 1790, 1149–1160. [Google Scholar] [CrossRef] [Green Version]
- Golbidi, S.; Badran, M.; Laher, I. Diabetes and alpha lipoic acid. Front. Pharmacol. 2011, 2, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Gomes, M.B.; Negrato, A. Alpha-lipoic acid as a pleiotropic compound with potential therapeutic use in diabetes and other chronic diseases. Diabetol. Metab. Syndr. 2014, 6, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baynest, H.W. Classification, pathophysiology, diagnosis and management of diabetes mellitus. J. Diabetes Metab. 2015, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Safdar, R.; Omar, A.A.; Arunagiri, A.; Regupathi, I. Potential of chitosan and its derivatives for controlled drug release applications—A review. J. Drug Deliv. Sci. Technol. 2019, 49, 642–659. [Google Scholar] [CrossRef]
- Kost, J.; Langer, R. Responsive polymeric delivery systems. Adv.Drug Deliv. Rev. 2001, 46, 125–148. [Google Scholar] [CrossRef]
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef]
- Constantin, S.M.; Buron, F.; Routier, S.; Vasincu, I.M.; Apotrosoaei, M.; Lupașcu, F.; Confederat, L.; Tuchiluș, C.; Constantin, M.T.; Sava, A.; et al. Formulation and characterization of new polymeric systems based on chitosan and xanthine derivatives with thiazolidine-4-one scaffold. Materials 2019, 12, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Shukr, M.H.; Ismail, S.; Ahmed, S.M. Development and optimization of ezetimib nanoparticles with improved antihyperlipidemic activity. J. Drug Deliv. Sci. Technol. 2019, 49, 383–395. [Google Scholar] [CrossRef]
- Lupascu, F.G.; Avram, I.; Confederat, L.; Constantin, S.M.; Stan, C.I.; Lupusoru, C.E.; Sava, A.; Profire, L. Biological evaluation of chitosan-antidiabetic drug formulations for the treatment of diabetes mellitus. Farmacia 2017, 65, 508–514. [Google Scholar]
- Naskar, S.; Sharma, S.; Kuotsu, K. Chitosan-based nanoparticles: An overview of biomedical applications and its preparation. J. Drug Deliv. Sci. Technol. 2019, 49, 66–81. [Google Scholar] [CrossRef]
- Confederat, L.; Motrescu, I.; Constantin, S.; Lupașcu, F.; Profire, L. Biopolymers with medical applications – Optimized method for obtaining chitosan-based delivery systems. Rev. Chim. 2018, 69, 1756–1759. [Google Scholar] [CrossRef]
- Papadimitriou, S.; Bikiaris, D. Novel self-assembled core-shell nanoparticles based on crystalline amorphous moieties of aliphatic polyesters for efficient controlled drug release. J. Control. Release 2009, 138, 174–189. [Google Scholar] [CrossRef] [PubMed]
- Aher, A.; Jain, H.K. Development and Validation of RP-HPLC method for estimation of Gliclazide in bulk and tablet dosage form. Am. J. Pharm. Tech. Res. 2015, 5, 658–667. [Google Scholar]
- Ezhilarasi, K.; Ramachandran, G.; Umapathy, D.; Rajaram, R.; Padmalayam, I.; Kumar, H.A. A simple and specific method for estimation of lipoic acid in human plasma by high performance liquid chromatography. J. Chromatogr. Sep. Tech. 2014, 5, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.S.; Tang, S.L.; Chiang, C.H.; Hosseinkhani, H.; Hong, P.-D.; Yeh, M.-K. Characterization and anti-tumour effects of chondroitin sulphate—Chitosan nanoparticles delivery systems. J. Nanopart. Res. 2014, 16, 2672–2687. [Google Scholar] [CrossRef]
- Dhanasingh, S.; Nallaperumal, S.K. Chitosan/Casein microparticles: Preparation, characterization and drug release studies. World Acad. Sci. Eng. Technol. 2010, 4, 180–184. [Google Scholar]
- Lupașcu, F.G.; Dash, M.; Samal, S.M.; Dubruel, P.; Lupusoru, C.E.; Lupusoru, R.V.; Profire, L. Development, optimization and biological evaluation of chitosan scaffold formulations of new xanthine derivatives for treatment of type 2 diabetes mellitus. Eur. J. Pharm. Sci. 2015, 77, 122–134. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Oprea, A.M.; Nistor, M.T.; Popa, M.I.; Lupusoru, C.E.; Vasile, C. In vitro and in vivo theophylline release from cellulose/chondroitin sulphate hydrogels. Carbohydr. Polym. 2012, 90, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.K.; Tripathi, P.; Ubaidulla, U.; Anand, V. Design and development of gliclazidemucoadhesivemicrocapsules: In vitro and in vivo evaluation. AAPS Pharm. Sci. Tech. 2008, 9, 224–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dressman, J. Evolution of dissolution media over the last twenty years. Dissolution Technol. 2014, 21, 6–10. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Confederat, L.-G.; Motrescu, I.; Condurache, M.I.; Constantin, S.; Bujor, A.; Tuchilus, C.G.; Profire, L. Chitosan-Based Delivery Systems Loaded with Glibenclamide and Lipoic Acid: Formulation, Characterization, and Kinetic Release Studies. Appl. Sci. 2020, 10, 7532. https://doi.org/10.3390/app10217532
Confederat L-G, Motrescu I, Condurache MI, Constantin S, Bujor A, Tuchilus CG, Profire L. Chitosan-Based Delivery Systems Loaded with Glibenclamide and Lipoic Acid: Formulation, Characterization, and Kinetic Release Studies. Applied Sciences. 2020; 10(21):7532. https://doi.org/10.3390/app10217532
Chicago/Turabian StyleConfederat, Luminita-Georgeta, Iuliana Motrescu, Mihaela Iustina Condurache, Sandra Constantin, Alexandra Bujor, Cristina Gabriela Tuchilus, and Lenuta Profire. 2020. "Chitosan-Based Delivery Systems Loaded with Glibenclamide and Lipoic Acid: Formulation, Characterization, and Kinetic Release Studies" Applied Sciences 10, no. 21: 7532. https://doi.org/10.3390/app10217532