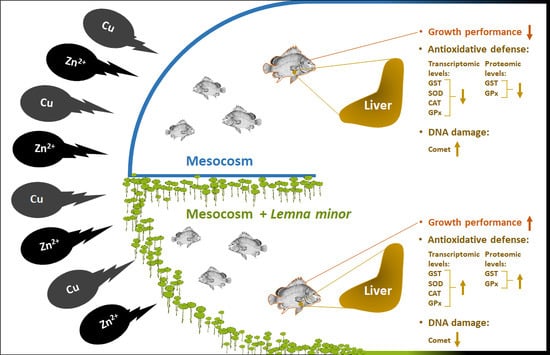
The Duckweed, Lemna minor Modulates Heavy Metal-Induced Oxidative Stress in the Nile Tilapia, Oreochromis niloticus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish and Mesocosm
2.2. Experimental Design
2.3. Growth Performance
2.4. Analysis of DNA
The Comet Assay
2.5. Gene Expression Analysis
2.5.1. RNA Extraction
2.5.2. Reverse Transcription (RT) Reaction
2.5.3. Quantitative Real Time-Polymerase Chain Reaction (qRT-PCR)
2.6. Biochemical Measurements
2.6.1. Glutathione-S-Transferase (GST) Activity
2.6.2. Glutathione Peroxidase (GPx) Activity
2.7. Statistical Analysis
3. Results
3.1. Effect of Duckweed on Growth Performance
3.2. Effect of Duckweed against Heavy Metals Induced DNA Damage
3.3. Effect of Duckweed on Antioxidants Gene Expression
3.4. Effect of Duckweed on the GST and GPx Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guerriero, G.; Parisi, C.; Abdel-Gawad, F.K.; Hentati, O.; D’Errico, G. Seasonal and pharmaceutical-induced changes in selenoprotein glutathione peroxidase 4 activity in the reproductive dynamics of the soil biosentinel Podarcis sicula (Chordata: Reptilia). Mol. Reprod. Dev. 2019, 86, 1378–1387. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, S.; Habauzit, D.; Charlier, T.; Pakdel, F. Emerging estrogenic pollutants in the aquatic environment and breast cancer. Genes 2017, 8, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fasulo, S.; Guerriero, G.; Cappello, S.; Colasanti, M.; Schettino, T.; Leonzio, C.; Mancini, G.; Gornati, R. The “SYSTEMS BIOLOGY” in the study of xenobiotic effects on marine organisms for evaluation of the environmental health status: Biotechnological applications for potential recovery strategies. Rev. Environ. Sci. Bio/Technol. 2015, 14, 339–345. [Google Scholar] [CrossRef]
- Diaconu, M.; Pavel, L.V.; Hlihor, R.-M.; Rosca, M.; Fertu, D.I.; Lenz, M.; Corvini, P.X.; Gavrilescu, M. Characterization of heavy metal toxicity in some plants and microorganisms—A preliminary approach for environmental bioremediation. New Biotechnol. 2020, 56, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Conte, B.; Sorbo, S.; Piscopo, M.; Rabbito, D.; De Ruberto, F.; Guerriero, G.; Basile, A. Antioxidant activity and ultrastructural alterations in the biosensor Lemna minor l. exposed in bags in sarno river (South Italy). Fresenius Environ. Bull. 2017, 26, 225–236. [Google Scholar]
- Sabiha, J.; Mehmood, T.; Chaudhry, M.M.; Tufail, M.; Irfan, N. Heavy metal pollution from phosphate rock used for the production of fertilizer in Pakistan. Microchem. J. 2009, 91, 94–99. [Google Scholar] [CrossRef]
- Jan, A.T.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q.M. Heavy metals and human health: Mechanistic insight into toxicity and counter defense system of antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef] [Green Version]
- Strungaru, S.-A.; Nicoara, M.; Teodosiu, C.; Baltag, E.; Ciobanu, C.; Plavan, G. Patterns of toxic metals bioaccumulation in a cross-border freshwater reservoir. Chemosphere 2018, 207, 192–202. [Google Scholar] [CrossRef]
- Abdel-Gawad, F.K.; Nassar, H.F.; Bassem, S.M.; Guerriero, G.; Khalil, W.K.B. Effect of polycyclic aromatic hydrocarbons (PAHs) on modulate genes encoding stress-related proteins and antioxidant enzymes in different marine fish species of Red Sea Water. World Appl. Sci. J. 2014, 32, 2337–2346. [Google Scholar] [CrossRef]
- Arena, C.; Vitale, L.; Bianchi, A.R.; Mistretta, C.; Vitale, E.; Parisi, C.; Guerriero, G.; Magliulo, V.; De Maio, A. The Ageing Process Affects the Antioxidant Defences and the Poly (ADPribosyl) ation Activity in Cistus Incanus L. Leaves. Antioxidants 2019, 8, 528. [Google Scholar] [CrossRef] [Green Version]
- Guerriero, G.; Trocchia, S.; Abdel-Gawad, F.K.; Ciarcia, G. Roles of reactive oxygen species in the spermatogenesis regulation. Front. Endocrinol. 2014, 5, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parisi, C.; Guerriero, G. Antioxidative Defense and Fertility Rate in the Assessment of Reprotoxicity Risk Posed by Global Warming. Antioxidants 2019, 8, 622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, A.A.-R.; El-Houseiny, W.; Abd Elhakeem, E.-M.; Ebraheim, L.L.; Ahmed, A.I.; Abd El-Hakim, Y.M. Effect of hexavalent chromium exposure on the liver and kidney tissues related to the expression of CYP450 and GST genes of Oreochromis niloticus fish: Role of curcumin supplemented diet. Ecotoxicol. Environ. Saf. 2020, 188, 109890. [Google Scholar] [CrossRef]
- Nofal, M.I. Effects of heavy metal pollution on nile tilapia in manzala farm: Oxidative stress biomarkers and histopathological findings. Int. J. Fish Aquat. Stud. 2019, 7, 315–328. [Google Scholar]
- Abdel-Tawwab, M.; El-Sayed, G.O.; Shady, S.H. Growth, biochemical variables, and zinc bioaccumulation in Nile tilapia, Oreochromis niloticus (L.) as affected by water-born zinc toxicity and exposure period. Int. Aquat. Res. 2016, 8, 197–206. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Al-Ogaily, S.M.; Al-Asgah, N.A.; Gropp, J. Effect of sublethal concentrations of copper on the growth performance of Oreochromis niloticus. J. Appl. Ichthyol. 2003, 19, 183–188. [Google Scholar] [CrossRef]
- Plavan, G.; Jitar, O.; Teodosiu, C.; Nicoara, M.; Micu, D.; Strungaru, S.-A. Toxic metals in tissues of fishes from the Black Sea and associated human health risk exposure. Environ. Sci. Pollut. Res. 2017, 24, 7776–7787. [Google Scholar] [CrossRef]
- Ahluwalia, S.S.; Goyal, D. Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour. Technol. 2007, 98, 2243–2257. [Google Scholar] [CrossRef]
- Hussein, H.; Farag, S.; Moawad, H. Isolation and characterization of Pseudomonas resistant to heavy metals contaminants. Arab J. Biotechnol. 2003, 7, 13–22. [Google Scholar]
- Kumar, V.; Parihar, R.D.; Sharma, A.; Bakshi, P.; Sidhu, G.P.S.; Bali, A.S.; Karaouzas, I.; Bhardwaj, R.; Thukral, A.K.; Gyasi-Agyei, Y. Global evaluation of heavy metal content in surface water bodies: A meta-analysis using heavy metal pollution indices and multivariate statistical analyses. Chemosphere 2019, 236, 124364. [Google Scholar] [CrossRef]
- Suhag, A.; Gupta, R.; Tiwari, A. Biosorptive removal of heavy metals from waste water using duckweed. Int. J. Biomed. Adv. Res. 2011, 2, 281–290. [Google Scholar] [CrossRef] [Green Version]
- Danner, R.I.; Mankasingh, U.; Anamthawat-Jonsson, K.; Thorarinsdottir, R.I. Designing aquaponic production systems towards integration into greenhouse farming. Water 2019, 11, 2123. [Google Scholar] [CrossRef] [Green Version]
- Zimmo, O.R.; Van Der Steen, N.P.; Gijzen, H.J. Effect of organic surface load on process performance of pilot-scale algae and duckweed-based waste stabilization ponds. J. Environ. Eng. 2005, 131, 587–594. [Google Scholar] [CrossRef]
- Organization for Economic Cooperation and Development. 221: Lemna sp. growth inhibition test. In OECD Guidelines for the Testing of Chemicals; Organization for Economic Cooperation and Development: Paris, France, 2006. [Google Scholar]
- Liu, C.; Gu, W.; Dai, Z.; Li, J.; Jiang, H.; Zhang, Q. Boron accumulation by Lemna minor L. under salt stress. Sci. Rep. 2018, 8, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Beheary, M.; M Sheta, B.; Hussein, M.; Nawareg, M.; A El-Matary, F.; Hyder, A. Environmental Remediation of Tilapia Aquaculture Wastewater Using Ceratophyllum demersum and Lemna minor. Egypt. J. Aquat. Biol. Fish. 2019, 23, 379–396. [Google Scholar] [CrossRef] [Green Version]
- Nassar, H.; Shaban, M.; Bassem, S.; Abdel-Gawad, F. Utilization of duckweed (DW) in nutrient removal from agricultural waste water and producing alternative economic animal fodder. Der Pharma Chem. 2015, 7, 280–285. [Google Scholar]
- Nieder, W.C.; Barnaba, E.; Findlay, S.E.; Hoskins, S.; Holochuck, N.; Blair, E.A. Distribution and abundance of submerged aquatic vegetation and Trapa natans in the Hudson River estuary. J. Coast. Res. 2004, 150–161. [Google Scholar] [CrossRef]
- Dirilgen, N.; Inel, Y. Effects of zinc and copper on growth and metal accumulation in duckweed, Lemna minor. Bull. Environ. Contam. Toxicol. 1994, 53. [Google Scholar] [CrossRef]
- Zayed, A.; Gowthaman, S.; Terry, N. Phytoaccumulation of trace elements by wetland plants: I. Duckweed. J. Environ. Qual. 1998, 27, 715–721. [Google Scholar] [CrossRef]
- Megateli, S.; Semsari, S.; Couderchet, M. Toxicity and removal of heavy metals (cadmium, copper, and zinc) by Lemna gibba. Ecotoxicol. Environ. Saf. 2009, 72, 1774–1780. [Google Scholar] [CrossRef]
- Kastratović, V.; Jaćimović, Ž.; DJurović, D.; Bigović, M.; Krivokapić, S. Lemna minor L.: As bioindicator of heavy metal pollution in Skadar lake: Montenegro. Kragujev. J. Sci. 2015, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Appenroth, K.-J.; Krech, K.; Keresztes, Á.; Fischer, W.; Koloczek, H. Effects of nickel on the chloroplasts of the duckweeds Spirodela polyrhiza and Lemna minor and their possible use in biomonitoring and phytoremediation. Chemosphere 2010, 78, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Oliveira, R.S.; Freitas, H.; Zhang, C. Biochemical and Molecular Mechanisms of Plant-Microbe-Metal Interactions: Relevance for Phytoremediation. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, G.; Ciarcia, G. Stress biomarkers and reproduction in fish. Fish Endocrinol. 2006, 2, 665–692. [Google Scholar] [CrossRef] [Green Version]
- Kroon, F.; Streten, C.; Harries, S. A protocol for identifying suitable biomarkers to assess fish health: A systematic review. PLoS ONE 2017, 12, e0174762. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Gawad, F.K.; Guerriero, G.; Khalil, W.K.; Abbas, H.H. Evaluation of oxidative stress, genotoxicity and gene expression alterations as oil pollution markers in Solea vulgaris, from Suez canal. Quantum Matter 2016, 5, 291–296. [Google Scholar] [CrossRef]
- Jitar, O.; Teodosiu, C.; Nicoara, M.; Plavan, G. Study of heavy metal pollution and bioaccumulation in the Black Sea living environment. Environ. Eng. Manag. J. 2013, 12, 271–276. [Google Scholar]
- Wang, J.; Xiao, J.; Zhang, J.; Chen, H.; Li, D.; Li, L.; Cao, J.; Xie, L.; Luo, Y. Effects of dietary Cu and Zn on the accumulation, oxidative stress and the expressions of immune-related genes in the livers of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2020. [Google Scholar] [CrossRef]
- Abd-Allah, M.M.; Ramadan, A.A.; Said, N.M.; Ibrahim, I.H.; Abdel-karim, E.A. Effects of Cadmium Chloride and Glyphosate on Antioxidants as Biochemical Biomarkers in Nile Tilapia. J. Aquac. Res. Dev. 2019, 10, 2. [Google Scholar] [CrossRef]
- Guerriero, G.; Di Finizio, A.; Ciarcia, G. Oxidative Defenses in the Sea Bass, Dicentrarchus labrax. In Oxygen Transport to Tissue XXIV; Dunn, J.F., Swartz, H.M., Eds.; Springer: Boston, MA, USA, 2003; pp. 681–688. ISBN 978-1-4613-4912-9. [Google Scholar]
- Yuan, S.-S.; Lv, Z.-M.; Zhu, A.-Y.; Zheng, J.-L.; Wu, C.-W. Negative effect of chronic cadmium exposure on growth, histology, ultrastructure, antioxidant and innate immune responses in the liver of zebrafish: Preventive role of blue light emitting diodes. Ecotoxicol. Environ. Saf. 2017, 139, 18–26. [Google Scholar] [CrossRef]
- Mohamed, A.; El Safty, M. Current situation of water pollution and its effect on aquatic life in Egypt. Egypt. J. Occup. Med. 2013, 37, 95–115. [Google Scholar] [CrossRef]
- Alawy, A.E.; El-Tras, W.F.; El Raiy, H.R. Impact of industrial wastewater on water and fish quality of Nile River in Kafr El-Zayat, Egypt. Benha Vet. Med. J. 2015, 28, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Blasiak, J.; Arabski, M.; Krupa, R.; Wozniak, K.; Zadrozny, M.; Kasznicki, J.; Zurawska, M.; Drzewoski, J. DNA damage and repair in type 2 diabetes mellitus. Mutat. Res./Fundam. Mol. Mech. Mutagenesis 2004, 554, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Khalil, W.K.B.; Weiler, U.; Becker, K. Influences of incorporating detoxified Jatropha curcas kernel meal in common carp (Cyprinus carpio L.) diet on the expression of growth hormone-and insulin-like growth factor-1-encoding genes. J. Anim. Physiol. Anim. Nutr. 2013, 97, 97–108. [Google Scholar] [CrossRef]
- AbdEl-Rahim, W.M.; Khalil, W.K.; Eshak, M.G. Evaluation of the gene expression changes in Nile tilapia (Oreochromis niloticus) as affected by the bio-removal of toxic textile dyes from aqueous solution in small-scale bioreactor. Environmentalist 2010, 30, 242–253. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar]
- Mannervik, B. Glutathione peroxidase. In Methods in Enzymology; Academic Press: New York, NY, USA, 1985; pp. 490–495. ISSN 0076-6879. [Google Scholar]
- Flohé, L.; Günzler, W.A. [12] Assays of glutathione peroxidase. In Methods in Enzymology; Academic Press: New York, NY, USA, 1984; pp. 114–120. ISSN 0076-6879. [Google Scholar]
- Shalaby, B.; Samy, Y.M.; Mashaly, A.O.; El Hefnawy, M.A.A. Comparative Geochemical Assessment of Heavy Metal Pollutants among the Mediterranean Deltaic Lakes Sediments (Edku, Burullus and Manzala), Egypt. Egypt. J. Chem. 2017, 60, 361–378. [Google Scholar] [CrossRef] [Green Version]
- Heath, A.G. Behavior and nervous system function. In Water Pollution and Fish Physiology; CRC Press: Boca Raton, FL, USA, 1987; pp. 181–196. [Google Scholar]
- Atli, G.; Alptekin, Ö.; Tükel, S.; Canli, M. Response of catalase activity to Ag+, Cd2+, Cr6+, Cu2+ and Zn2+ in five tissues of freshwater fish Oreochromis niloticus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2006, 143, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Saglam, D.; Atli, G.; Dogan, Z.; Baysoy, E.; Gurler, C.; Eroglu, A.; Canli, M. Response of the antioxidant system of freshwater fish (Oreochromis niloticus) exposed to metals (Cd, Cu) in differing hardness. Turk. J. Fish. Aquat. Sci. 2014, 14, 43–52. [Google Scholar] [CrossRef]
- Rae, T.D.; Schmidt, P.J.; Pufahl, R.A.; Culotta, V.C.; O’Halloran, T.V. Undetectable intracellular free copper: The requirement of a copper chaperone for superoxide dismutase. Science 1999, 284, 805–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliwell, B.; Gutteridge, J.M.C. Role of free radicals and catalytic metal ions in human disease: An overview. In Oxygen Radicals in Biological Systems Part B: Oxygen Radicals and Antioxidants; Methods in Enzymology; Academic Press: New York, NY, USA, 1990; pp. 1–85. [Google Scholar]
- McGeer, J.C.; Szebedinszky, C.; McDonald, D.G.; Wood, C.M. Effects of chronic sublethal exposure to waterborne Cu, Cd or Zn in rainbow trout. 1: Iono-regulatory disturbance and metabolic costs. Aquat. Toxicol. 2000, 50, 231–243. [Google Scholar] [CrossRef]
- Trevisan, R.; Flesch, S.; Mattos, J.J.; Milani, M.R.; Bainy, A.C.D.; Dafre, A.L. Zinc causes acute impairment of glutathione metabolism followed by coordinated antioxidant defenses amplification in gills of brown mussels Perna perna. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2014, 159, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Omar, W.A.; Mikhail, W.Z.; Abdo, H.M.; Abou El Defan, T.A.; Poraas, M.M. Ecological risk assessment of metal pollution along greater Cairo sector of the river Nile, Egypt, using nile tilapia, Oreochromis niloticus, as Bioindicator. J. Toxicol. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Xiong, D.; Fang, T.; Yu, L.; Sima, X.; Zhu, W. Effects of nano-scale TiO2, ZnO and their bulk counterparts on zebrafish: Acute toxicity, oxidative stress and oxidative damage. Sci. Total Environ. 2011, 409, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Ali, D.; Ali, H. Susceptibility of the freshwater pulmonate snail Lymnea luteola L. to copper oxide nanoparticle. Toxicol. Environ. Chem. 2015, 97, 576–587. [Google Scholar] [CrossRef]
- Alkaladi, A.; Afifi, M.; Mosleh, Y.; AbuZinada, O. Histopathological effects of zinc oxide nanoparticles on the liver and gills of Oreochromis niloticus, protective effect of vitamins C and E. Microbiol 2014, 8, 4549–4558. [Google Scholar]
- Hansen, B.H.; Rømma, S.; Garmo, Ø.A.; Pedersen, S.A.; Olsvik, P.A.; Andersen, R.A. Induction and activity of oxidative stress-related proteins during waterborne Cd/Zn-exposure in brown trout (Salmo trutta). Chemosphere 2007, 67, 2241–2249. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-Y.; Lee, S.Y.; Cho, Y.S.; Bang, I.C.; Kim, K.H.; Kim, D.S.; Nam, Y.K. Molecular characterization and mRNA expression during metal exposure and thermal stress of copper/zinc- and manganese-superoxide dismutases in disk abalone, Haliotis discus discus. Fish Shellfish Immunol. 2007, 23, 1043–1059. [Google Scholar] [CrossRef] [PubMed]
- Craig, P.M.; Wood, C.M.; McClelland, G.B. Oxidative stress response and gene expression with acute copper exposure in zebrafish (Danio rerio). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R1882–R1892. [Google Scholar] [CrossRef] [Green Version]
- Varela-Valencia, R.; Gómez-Ortiz, N.; Oskam, G.; de Coss, R.; Rubio-Piña, J.; del Río-García, M.; Albores-Medina, A.; Zapata-Perez, O. The effect of titanium dioxide nanoparticles on antioxidant gene expression in tilapia (Oreochromis niloticus). J. Nanoparticle Res. 2014, 16, 2369. [Google Scholar] [CrossRef]
- El-Sayed, Y.S.; El-Gazzar, A.M.; El-Nahas, A.F.; Ashry, K.M. Vitamin C modulates cadmium-induced hepatic antioxidants’ gene transcripts and toxicopathic changes in Nile tilapia, Oreochromis niloticus. Environ. Sci. Pollut. Res. 2016, 23, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Obiakor, M.O.; Okonkwo, J.C.; Ezeonyejiaku, C.D.; Ezenwelu, C.O. Genotoxicology: Single and joint action of copper and zinc to Synodontis clarias and Tilapia nilotica. J. Appl. Sci. Environ. Manag. 2010, 14. [Google Scholar] [CrossRef] [Green Version]
- Shahzad, K.; Khan, M.N.; Jabeen, F.; Kosour, N.; Chaudhry, A.S.; Sohail, M.; Ahmad, N. Toxicity of zinc oxide nanoparticles (ZnO-NPs) in tilapia (Oreochromis mossambicus): Tissue accumulation, oxidative stress, histopathology and genotoxicity. Int. J. Environ. Sci. Technol. 2019, 16, 1973–1984. [Google Scholar] [CrossRef]
- Shahzad, K.; Khan, M.N.; Jabeen, F.; Kosour, N.; Chaudhry, A.S.; Sohail, M. Evaluating toxicity of copper (II) oxide nanoparticles (CuO-NPs) through waterborne exposure to tilapia (Oreochromis mossambicus) by tissue accumulation, oxidative stress, histopathology, and genotoxicity. Environ. Sci. Pollut. Res. 2018, 25, 15943–15953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Maio, A.; Trocchia, S.; Guerriero, G. The amphibian Pelophylax bergeri (Günther, 1986) testis poly (ADP-ribose) polymerases: Relationship to endocrine disruptors during spermatogenesis. Ital. J. Zool. 2014, 81, 256–263. [Google Scholar] [CrossRef] [Green Version]
- Scalici, M.; Traversetti, L.; Spani, F.; Malafoglia, V.; Colamartino, M.; Persichini, T.; Cappello, S.; Mancini, G.; Guerriero, G.; Colasanti, M. Shell fluctuating asymmetry in the sea-dwelling benthic bivalve Mytilus galloprovincialis (Lamarck, 1819) as morphological markers to detect environmental chemical contamination. Ecotoxicology 2017, 26, 396–404. [Google Scholar] [CrossRef]


| Gene | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) |
|---|---|---|
| SOD | GGTGCCCTGGAGCCCTA | ATGCGAAGTCTTCCACTGTC |
| CAT | TCCTGAATGAGGAGGAGCGA | ATCTTAGATGAGGCGGTGATG |
| GPx | CCAAGAGAACTGCAAGAACGA | CAGGACACGTCATTCCTACAC |
| GST | TAATGGGAGAGGGAAGATGG | CTCTGCGATGTAATTCAGGA |
| β-actin | CAATGAGAGGTTCCGTTGC | AGGATTCCATACCAAGGAAGG |
| Treatment | Initial Weight (g) | Final Weight (g) |
|---|---|---|
| Control | 36.2 ± 2.4 | 99.3 ± 3.2 a |
| CuL | 37.1 ± 3.2 | 88.1 ± 4.1 ab |
| CuH | 35.4 ± 1.9 | 77.6 ± 2.9 bc |
| CuL + L. minor | 38.2 ± 2.7 | 93.2 ± 4.8 ab |
| CuH + L. minor | 36.4 ± 1.6 | 84.4 ± 5.2 b |
| ZnL | 36.2 ± 1.5 | 81.5 ± 3.7 b |
| ZnH | 37.5 ± 2.2 | 71.2 ± 2.4 c |
| ZnL + L. minor | 38.2 ± 3.3 | 89.1 ± 3.8 ab |
| ZnH + L. minor | 36.6 ± 2.1 | 80.3 ± 3.1 b |
| Treatment | Total Comets | Comet Class | DNA % Damaged Cells | |||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |||
| Control | 33 | 467 | 22 | 11 | 0 | 6.6 ± 1.1 c |
| CuL | 46 | 454 | 17 | 16 | 13 | 9.2 ± 1.6 bc |
| CuH | 87 | 413 | 26 | 32 | 29 | 17.4 ± 2.4 a |
| CuL + L. minor | 38 | 462 | 12 | 15 | 11 | 7.6 ± 1.2 c |
| CuH + L. minor | 56 | 444 | 16 | 21 | 19 | 11.2 ± 1.6 b |
| ZnL | 49 | 451 | 18 | 15 | 16 | 9.8 ± 1.5 bc |
| ZnH | 98 | 402 | 28 | 37 | 33 | 19.6 ± 2.2 a |
| ZnL + L. minor | 39 | 461 | 21 | 11 | 7 | 7.8 ± 1.3 c |
| ZnH + L. minor | 62 | 438 | 18 | 22 | 21 | 12.4 ± 1.8 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Gawad, F.K.; Khalil, W.K.B.; Bassem, S.M.; Kumar, V.; Parisi, C.; Inglese, S.; Temraz, T.A.; Nassar, H.F.; Guerriero, G. The Duckweed, Lemna minor Modulates Heavy Metal-Induced Oxidative Stress in the Nile Tilapia, Oreochromis niloticus. Water 2020, 12, 2983. https://doi.org/10.3390/w12112983
Abdel-Gawad FK, Khalil WKB, Bassem SM, Kumar V, Parisi C, Inglese S, Temraz TA, Nassar HF, Guerriero G. The Duckweed, Lemna minor Modulates Heavy Metal-Induced Oxidative Stress in the Nile Tilapia, Oreochromis niloticus. Water. 2020; 12(11):2983. https://doi.org/10.3390/w12112983
Chicago/Turabian StyleAbdel-Gawad, Fagr Kh., Wagdy K. B. Khalil, Samah M. Bassem, Vikas Kumar, Costantino Parisi, Sara Inglese, Tarek A. Temraz, Hossam F. Nassar, and Giulia Guerriero. 2020. "The Duckweed, Lemna minor Modulates Heavy Metal-Induced Oxidative Stress in the Nile Tilapia, Oreochromis niloticus" Water 12, no. 11: 2983. https://doi.org/10.3390/w12112983