Systemically Delivered Magnetic Hyperthermia for Prostate Cancer Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Zn- and Mn-Doped Iron Oxide Nanoparticles
2.3. Characterization of Nanoparticles
2.4. Preparation of Nanoclusters
2.5. Preparation of SiNc-Loaded Nanoclusters
2.6. Characterization of Nanoclusters
2.7. In Vitro Studies
2.8. In Vivo Studies
2.8.1. Development of a Mouse Model of Human Prostate Cancer
2.8.2. Evaluation of Nanocluster Biodistribution
2.8.3. Assessment of the Therapeutic Efficacy of Nanocluster-Mediated Hyperthermia
2.9. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of Nanoclusters
3.2. In Vitro Evaluation of Nanoclusters
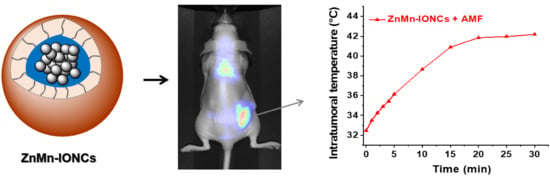
3.3. In Vivo Evaluation of Nanoclusters
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Litwin, M.S.; Tan, H.J. The Diagnosis and Treatment of Prostate Cancer: A Review. JAMA 2017, 317, 2532–2542. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.C. Treatment Options for Hormone-Refractory Prostate Cancer. Rev. Urol. 2007, 9 (Suppl. 2), S13–S18. [Google Scholar] [PubMed]
- Deatsch, A.E.; Evans, B.A. Heating Efficiency in Magnetic Nanoparticle Hyperthermia. J. Magn. Magn. Mater. 2014, 354, 163–172. [Google Scholar] [CrossRef]
- Torres-Lugo, M.; Rinaldi, C. Thermal Potentiation of Chemotherapy by Magnetic Nanoparticles. Nanomedicine 2013, 8, 1689–1707. [Google Scholar] [CrossRef] [Green Version]
- Kumar, C.S.; Mohammad, F. Magnetic Nanomaterials for Hyperthermia-Based Therapy and Controlled Drug Delivery. Adv. Drug Deliv. Rev. 2011, 63, 789–808. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudi, K.; Bouras, A.; Bozec, D.; Ivkov, R.; Hadjipanayis, C. Magnetic Hyperthermia Therapy for the Treatment of Glioblastoma: A Review of the Therapy’s History, Efficacy and Application in Humans. Int. J. Hyperth. 2018, 34, 1316–1328. [Google Scholar] [CrossRef] [Green Version]
- Attaluri, A.; Kandala, S.K.; Wabler, M.; Zhou, H.; Cornejo, C.; Armour, M.; Hedayati, M.; Zhang, Y.; DeWeese, T.L.; Herman, C.; et al. Magnetic Nanoparticle Hyperthermia Enhances Radiation Therapy: A Study in Mouse Models of Human Prostate Cancer. Int. J. Hyperth. 2015, 31, 359–374. [Google Scholar] [CrossRef] [Green Version]
- Chang, D.; Lim, M.; Goos, J.; Qiao, R.; Ng, Y.Y.; Mansfeld, F.M.; Jackson, M.; Davis, T.P.; Kavallaris, M. Biologically Targeted Magnetic Hyperthermia: Potential and Limitations. Front. Pharmacol. 2018, 9, 831. [Google Scholar] [CrossRef] [Green Version]
- Toraya-Brown, S.; Sheen, M.R.; Zhang, P.; Chen, L.; Baird, J.R.; Demidenko, E.; Turk, M.J.; Hoopes, P.J.; Conejo-Garcia, J.R.; Fiering, S. Local Hyperthermia Treatment of Tumors Induces CD8(+) T Cell-Mediated Resistance Against Distal and Secondary Tumors. Nanomedicine 2014, 10, 1273–1285. [Google Scholar] [CrossRef] [Green Version]
- Moy, A.J.; Tunnell, J.W. Combinatorial Immunotherapy and Nanoparticle Mediated Hyperthermia. Adv. Drug Deliv. Rev. 2017, 114, 175–183. [Google Scholar] [CrossRef]
- Johannsen, M.; Thiesen, B.; Wust, P.; Jordan, A. Magnetic Nanoparticle Hyperthermia for Prostate Cancer. Int. J. Hyperth. 2010, 26, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, M.; Gneveckow, U.; Taymoorian, K.; Thiesen, B.; Waldofner, N.; Scholz, R.; Jung, K.; Jordan, A.; Wust, P.; Loening, S.A. Morbidity and Quality of Life During Thermotherapy Using Magnetic Nanoparticles in Locally Recurrent Prostate Cancer: Results of a Prospective Phase I Trial. Int. J. Hyperth. 2007, 23, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, M.; Gneveckow, U.; Thiesen, B.; Taymoorian, K.; Cho, C.H.; Waldofner, N.; Scholz, R.; Jordan, A.; Loening, S.A.; Wust, P. Thermotherapy of Prostate Cancer Using Magnetic Nanoparticles: Feasibility, Imaging, and Three-Dimensional Temperature Distribution. Eur. Urol. 2007, 52, 1653–1661. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Wang, L.F.; Ding, W.J.; Zhou, M.J.; Jin, H.K.; Su, S.F.; Ouyang, W.W. Clinical Trials of Magnetic Induction Hyperthermia for Treatment of Tumours. OA Cancer 2014, 2, 2. [Google Scholar]
- Shetake, N.G.; Balla, M.M.S.; Kumar, A.; Pandey, B.N. Magnetic Hyperthermia Therapy: An Emerging Modality of Cancer Treatment in Combination with Radiotherapy. J. Radiat. Cancer Res. 2016, 7, 13–17. [Google Scholar]
- Albarqi, H.A.; Wong, L.H.; Schumann, C.; Sabei, F.Y.; Korzun, T.; Li, X.; Hansen, M.N.; Dhagat, P.; Moses, A.S.; Taratula, O.; et al. Biocompatible Nanoclusters with High Heating Efficiency for Systemically Delivered Magnetic Hyperthermia. ACS Nano 2019, 13, 6383–6395. [Google Scholar] [CrossRef]
- Huang, H.S.; Hainfeld, J.F. Intravenous Magnetic Nanoparticle Cancer Hyperthermia. Int. J. Nanomed. 2013, 8, 2521–2532. [Google Scholar] [CrossRef] [Green Version]
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Wuyts, F.; Maes, L. Cobalt Toxicity in Humans-A Review of the Potential Sources and Systemic Health Effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef]
- Jeun, M.; Moon, S.J.; Kobayashi, H.; Shin, H.Y.; Tomitaka, A.; Kim, Y.J.; Takemura, Y.; Paek, S.H.; Park, K.H.; Chung, K.-W. Effects of Mn Concentration on the AC Magnetically Induced Heating Characteristics of Superparamagnetic Mn x Zn 1− x Fe2O4 nanoparticles for Hyperthermia. Appl. Phys. Lett. 2010, 96, 202511. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H. Size-Controlled Synthesis of Magnetite Nanoparticles. J. Am. Chem. Soc. 2002, 124, 8204–8205. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Wang, S.X.; Li, G. Monodisperse MFe2O4 (M = Fe, Co, Mn) Nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Taratula, O.; Schumann, C.; Naleway, M.A.; Pang, A.J.; Chon, K.J.; Taratula, O. A Multifunctional Theranostic Platform Based on Phthalocyanine-Loaded Dendrimer for Image-Guided Drug Delivery and Photodynamic Therapy. Mol. Pharm. 2013, 10, 3946–3958. [Google Scholar] [CrossRef] [PubMed]
- Dani, R.K.; Schumann, C.; Taratula, O.; Taratula, O. Temperature-Tunable Iron Oxide Nanoparticles for Remote-Controlled Drug Release. AAPS PharmSciTech 2014, 15, 963–972. [Google Scholar] [CrossRef] [Green Version]
- Schumann, C.; Chan, S.; Khalimonchuk, O.; Khal, S.; Moskal, V.; Shah, V.; Alani, A.W.; Taratula, O.; Taratula, O. Mechanistic Nanotherapeutic Approach Based on siRNA-Mediated DJ-1 Protein Suppression for Platinum-Resistant Ovarian Cancer. Mol. Pharm. 2016, 13, 2070–2083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duong, T.; Li, X.; Yang, B.; Schumann, C.; Albarqi, H.A.; Taratula, O.; Taratula, O. Phototheranostic Nanoplatform Based on a Single Cyanine Dye for Image-Guided Combinatorial Phototherapy. Nanomedicine 2017, 13, 955–963. [Google Scholar] [CrossRef]
- Taratula, O.; Dani, R.K.; Schumann, C.; Xu, H.; Wang, A.; Song, H.; Dhagat, P.; Taratula, O. Multifunctional Nanomedicine Platform for Concurrent Delivery of Chemotherapeutic Drugs and Mild Hyperthermia to Ovarian Cancer Cells. Int. J. Pharm. 2013, 458, 169–180. [Google Scholar] [CrossRef]
- Li, X.; Schumann, C.; Albarqi, H.A.; Lee, C.J.; Alani, A.W.G.; Bracha, S.; Milovancev, M.; Taratula, O.; Taratula, O. A Tumor-Activatable Theranostic Nanomedicine Platform for NIR Fluorescence-Guided Surgery and Combinatorial Phototherapy. Theranostics 2018, 8, 767–784. [Google Scholar] [CrossRef]
- Taratula, O.; Schumann, C.; Duong, T.; Taylor, K.L.; Taratula, O. Dendrimer-Encapsulated Naphthalocyanine as a Single Agent-Based Theranostic Nanoplatform for Near-Infrared Fluorescence Imaging and Combinatorial Anticancer Phototherapy. Nanoscale 2015, 7, 3888–3902. [Google Scholar] [CrossRef]
- Qu, Y.; Li, J.; Ren, J.; Leng, J.; Lin, C.; Shi, D. Enhanced Magnetic Fluid Hyperthermia by Micellar Magnetic Nanoclusters Composed of Mn(x)Zn(1-x)Fe(2)O(4) Nanoparticles for Induced Tumor Cell Apoptosis. ACS Appl. Mater. Interfaces 2014, 6, 16867–16879. [Google Scholar] [CrossRef]
- Lee, J.H.; Jang, J.T.; Choi, J.S.; Moon, S.H.; Noh, S.H.; Kim, J.W.; Kim, J.G.; Kim, I.S.; Park, K.I.; Cheon, J. Exchange-Coupled Magnetic Nanoparticles for Efficient Heat Induction. Nat. Nanotechnol. 2011, 6, 418–422. [Google Scholar] [CrossRef]
- Wang, N.; Liu, B.; Liang, L.; Wu, Y.; Xie, H.; Huang, J.; Guo, X.; Tan, J.; Zhan, X.; Liu, Y.; et al. Antiangiogenesis Therapy of Endometriosis Using PAMAM as a Gene Vector in a Noninvasive Animal Model. Biomed. Res. Int. 2014, 2014, 546479. [Google Scholar] [CrossRef] [PubMed]
- Bauer, L.M.; Situ, S.F.; Griswold, M.A.; Samia, A.C.S. High-Performance Iron Oxide Nanoparticles for Magnetic Particle Imaging–Guided Hyperthermia (hMPI). Nanoscale 2016, 8, 12162–12169. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.T.; Lee, J.; Seon, J.; Ju, E.; Kim, M.; Kim, Y.I.; Kim, M.G.; Takemura, Y.; Arbab, A.S.; Kang, K.W. Giant Magnetic Heat Induction of Magnesium-Doped γ-Fe2O3 Superparamagnetic Nanoparticles for Completely Killing Tumors. Adv. Mater. 2018, 30, 1704362. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.t.; Nah, H.; Lee, J.H.; Moon, S.H.; Kim, M.G.; Cheon, J. Critical Enhancements of MRI Contrast and Hyperthermic Effects by Dopant-Controlled Magnetic Nanoparticles. Angew. Chem. Int. Ed. 2009, 48, 1234–1238. [Google Scholar] [CrossRef]
- Hayashi, K.; Nakamura, M.; Sakamoto, W.; Yogo, T.; Miki, H.; Ozaki, S.; Abe, M.; Matsumoto, T.; Ishimura, K. Superparamagnetic Nanoparticle Clusters for Cancer Theranostics Combining Magnetic Resonance Imaging and Hyperthermia Treatment. Theranostics 2013, 3, 366–376. [Google Scholar] [CrossRef] [Green Version]
- Asem, H.; Zhao, Y.; Ye, F.; Barrefelt, A.; Abedi-Valugerdi, M.; El-Sayed, R.; El-Serafi, I.; Abu-Salah, K.M.; Hamm, J.; Muhammed, M.; et al. Biodistribution of Biodegradable Polymeric Nano-Carriers Loaded with Busulphan and Designed for Multimodal Imaging. J. Nanobiotechnol. 2016, 14, 82. [Google Scholar] [CrossRef] [Green Version]
- Moses, A.S.; Taratula, O.R.; Lee, H.; Luo, F.; Grenz, T.; Korzun, T.; Lorenz, A.S.; Sabei, F.Y.; Bracha, S.; Alani, A.W.G.; et al. Nanoparticle-Based Platform for Activatable Fluorescence Imaging and Photothermal Ablation of Endometriosis. Small 2020, 16, e1906936. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Li, X.; Taratula, O.; Taratula, O.; Schumann, C.; Minko, T. LHRH-Targeted Drug Delivery Systems for Cancer Therapy. Mini Rev. Med. Chem. 2017, 17, 258–267. [Google Scholar] [CrossRef]
- Tay, Z.W.; Chandrasekharan, P.; Chiu-Lam, A.; Hensley, D.W.; Dhavalikar, R.; Zhou, X.Y.; Yu, E.Y.; Goodwill, P.W.; Zheng, B.; Rinaldi, C.; et al. Magnetic Particle Imaging-Guided Heating in Vivo Using Gradient Fields for Arbitrary Localization of Magnetic Hyperthermia Therapy. ACS Nano 2018, 12, 3699–3713. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albarqi, H.A.; Demessie, A.A.; Sabei, F.Y.; Moses, A.S.; Hansen, M.N.; Dhagat, P.; Taratula, O.R.; Taratula, O. Systemically Delivered Magnetic Hyperthermia for Prostate Cancer Treatment. Pharmaceutics 2020, 12, 1020. https://doi.org/10.3390/pharmaceutics12111020
Albarqi HA, Demessie AA, Sabei FY, Moses AS, Hansen MN, Dhagat P, Taratula OR, Taratula O. Systemically Delivered Magnetic Hyperthermia for Prostate Cancer Treatment. Pharmaceutics. 2020; 12(11):1020. https://doi.org/10.3390/pharmaceutics12111020
Chicago/Turabian StyleAlbarqi, Hassan A., Ananiya A. Demessie, Fahad Y. Sabei, Abraham S. Moses, Mikkel N. Hansen, Pallavi Dhagat, Olena R. Taratula, and Oleh Taratula. 2020. "Systemically Delivered Magnetic Hyperthermia for Prostate Cancer Treatment" Pharmaceutics 12, no. 11: 1020. https://doi.org/10.3390/pharmaceutics12111020