Abstract
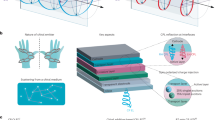
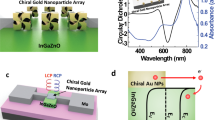
The biological effects of circularly polarized light on living cells are considered to be negligibly weak. Here, we show that the differentiation of neural stem cells into neurons can be accelerated by circularly polarized photons when DNA-bridged chiral assemblies of gold nanoparticles are entangled with the cells’ cytoskeletal fibres. By using cell-culture experiments and plasmonic-force calculations, we demonstrate that the nanoparticle assemblies exert a circularly-polarized-light-dependent force on the cytoskeleton, and that the light-induced periodic mechanical deformation of actin nanofibres with a frequency of 50 Hz stimulates the differentiation of neural stem cells into the neuronal phenotype. When implanted in the hippocampus of a mouse model of Alzheimer’s disease, neural stem cells illuminated following a polarity-optimized protocol reduced the formation of amyloid plaques by more than 70%. Our findings suggest that circularly polarized light can guide cellular development for biomedical use.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the paper and its Supplementary Information. The raw data are available from figshare with the identifier https://doi.org/10.6084/m9.figshare.12931874.
References
Paviolo, C. et al. Laser exposure of gold nanorods can increase neuronal cell outgrowth. Biotechnol. Bioeng. 110, 2277–2291 (2013).
Baba, J. S., Chung, J. R., DeLaughter, A. H., Cameron, B. D. & Cote, G. L. Development and calibration of an automated Mueller matrix polarization imaging system. J. Biomed. Opt. 7, 341–349 (2002).
Funck, T., Nicoli, F., Kuzyk, A. & Liedl, T. Sensing picomolar concentrations of RNA using switchable plasmonic chirality. Angew. Chem. Int. Ed. 57, 13495–13498 (2018).
Hendry, E. et al. Ultrasensitive detection and characterization of biomolecules using superchiral fields. Nat. Nanotechnol. 5, 783–787 (2010).
Chen, W. et al. Nanoparticle superstructures made by polymerase chain reaction: collective interactions of nanoparticles and a new principle for chiral materials. Nano Lett. 9, 2153–2159 (2009).
Zhao, X. et al. Tuning the interactions between chiral plasmonic films and living cells. Nat. Commun. 8, 2007 (2017).
Patel, M., Moon, H. J., Hong, J. H. & Jeong, B. Chiro-optical modulation for NURR1 production from stem cells. ACS Chem. Neurosci. 8, 1455–1458 (2017).
Morrow, S. M., Bissette, A. J. & Fletcher, S. P. Transmission of chirality through space and across length scales. Nat. Nanotechnol. 12, 410–419 (2017).
Vestler, D. et al. Circular dichroism enhancement in plasmonic nanorod metamaterials. Opt. Express 26, 17841–17848 (2018).
Govan, J. & Gun’ko, Y. K. in Nanoscience Vol. 3, 1–30 (Royal Society of Chemistry, 2016).
Auguié, B., Alonso-Gómez, J. L., Guerrero-Martínez, A. & Liz-Marzán, L. M. Fingers crossed: optical activity of a chiral dimer of plasmonic nanorods. J. Phys. Chem. Lett. 2, 846–851 (2011).
Sun, M. et al. Intracellular localization of nanoparticle dimers by chirality reversal. Nat. Commun. 8, 1847 (2017).
Ma, W. et al. Chiral inorganic nanostructures. Chem. Rev. 117, 8041–8093 (2017).
Zhou, L. A. et al. Quantifying hot carrier and thermal contributions in plasmonic photocatalysis. Science 362, 69–72 (2018).
Sun, M. Z. et al. Site-selective photoinduced cleavage and profiling of DNA by chiral semiconductor nanoparticles. Nat. Chem. 10, 821–830 (2018).
Li, S. et al. Single- and multi-component chiral supraparticles as modular enantioselective catalysts. Nat. Commun. 10, 4826 (2019).
Kim, J.-Y. et al. Assembly of gold nanoparticles into chiral superstructures driven by circularly polarized light. J. Am. Chem. Soc. 141, 11739–11744 (2019).
Valev, V. K. et al. Nanostripe length dependence of plasmon-induced material deformations. Opt. Lett. 38, 2256–2258 (2013).
Laramy, C. R., O’Brien, M. N. & Mirkin, C. A. Crystal engineering with DNA. Nat. Rev. Mater. 4, 201–224 (2019).
Chen, G. et al. Regioselective surface encoding of nanoparticles for programmable self-assembly. Nat. Mater. 18, 169–174 (2019).
Leung, K., Chakraborty, K., Saminathan, A. & Krishnan, Y. A DNA nanomachine chemically resolves lysosomes in live cells. Nat. Nanotechnol. 14, 176–183 (2019).
Zhang, Q. F. et al. Unraveling the origin of chirality from plasmonic nanoparticle–protein complexes. Science 365, 1475–1478 (2019).
Lu, D. R., Zhou, J. J., Chen, Y. H., Ma, J. L. & Duan, H. W. Self-assembly of polymer-coated plasmonic nanocrystals: from synthetic approaches to practical applications. Macromol. Rapid Commun. 40, 1800613 (2019).
Ben-Moshe, A., Maoz, B., Govorov, A. O. & Markovich, G. Chirality and chiroptical effects in inorganic nanocrystal systems with plasmon and exciton resonances. Chem. Soc. Rev. 42, 7028–7041 (2013).
Verma, A. et al. Surface-structure-regulated cell-membrane penetration by monolayer-protected nanoparticles. Nat. Mater. 7, 588–595 (2008).
Samanta, D., Ebrahimi, S. B. & Mirkin, C. A. Nucleic‐acid structures as intracellular probes for live cells. Adv. Mater. 32, 1901743 (2019).
Wang, Z. et al. Bioinspired nanocomplex for spatiotemporal imaging of sequential mRNA expression in differentiating neural stem cells. ACS Nano 8, 12386–12396 (2014).
Calza, L., Fernandez, M., Giuliani, A., Aloe, L. & Giardino, L. Thyroid hormone activates oligodendrocyte precursors and increases a myelin-forming protein and NGF content in the spinal cord during experimental allergic encephalomyelitis. Proc. Natl Acad. Sci. USA 99, 3258–3263 (2002).
Solanki, A., Shah, S., Yin, P. T. & Lee, K.-B. Nanotopography-mediated reverse uptake for siRNA delivery into neural stem cells to enhance neuronal differentiation. Sci. Rep. 3, 1553 (2013).
Johnson, G. V. W. & Jope, R. S. The role of microtubule-associated protein-2 (Map-2) in neuronal growth, plasticity and degeneration. J. Neurosci. Res. 33, 505–512 (1992).
Gu, H., Yu, S. P., Gutekunst, C.-A., Gross, R. E. & Wei, L. Inhibition of the Rho signaling pathway improves neurite outgrowth and neuronal differentiation of mouse neural stem cells. Int. J. Physiol. Pathophysiol. Pharmacol. 5, 11–20 (2013).
Garland, P., Quraishe, S., French, P. & O’Connor, V. Expression of the MAST family of senone/threonine kinases. Brain Res. 1195, 12–19 (2008).
Dupont, S. et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011).
Brusatin, G., Panciera, T., Gandin, A., Citron, A. & Piccolo, S. Biomaterials and engineered microenvironments to control YAP/TAZ-dependent cell behaviour. Nat. Mater. 17, 1063–1075 (2018).
Xue, X. F. et al. Mechanics-guided embryonic patterning of neuroectoderm tissue from human pluripotent stem cells. Nat. Mater. 17, 633–641 (2018).
Cui, Y. et al. Cyclic stretching of soft substrates induces spreading and growth. Nat. Commun. 6, 6333 (2015).
Asbury, C. L., Gestaut, D. R., Powers, A. F., Franck, A. D. & Davis, T. N. The Dam1 kinetochore complex harnesses microtubule dynamics to produce force and movement. Proc. Natl Acad. Sci. USA 103, 9873–9878 (2006).
Kreplak, L., Herrmann, H. & Aebi, U. Tensile properties of single desmin intermediate filaments. Biophys. J. 94, 2790–2799 (2008).
Guolla, L., Bertrand, M., Haase, K. & Pelling, A. E. Force transduction and strain dynamics in actin stress fibres in response to nanonewton forces. J. Cell Sci. 125, 603–613 (2012).
Shafrir, Y. & Forgacs, G. Mechanotransduction through the cytoskeleton. Am. J. Physiol. Cell Physiol. 282, C479–C486 (2002).
Juan, M. L., Righini, M. & Quidant, R. Plasmon nano-optical tweezers. Nat. Photon. 5, 349–356 (2011).
Karafyllidis, I. G. & Lagoudas, D. C. Microtubules as mechanical force sensors. Biosystems 88, 137–146 (2007).
Fletcher, D. A. & Mullins, R. D. Cell mechanics and the cytoskeleton. Nature 463, 485–492 (2010).
Torii, T. et al. Arf6 guanine-nucleotide exchange factor, cytohesin-2, interacts with actinin-1 to regulate neurite extension. Cell. Signal. 24, 1872–1882 (2012).
Tee, Y. H. et al. Cellular chirality arising from the self-organization of the actin cytoskeleton. Nat. Cell Biol. 17, 445–457 (2015).
Da Mesquita, S. et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 560, 185–191 (2018).
Rice, H. C. et al. Secreted amyloid-β precursor protein functions as a GABABR1a ligand to modulate synaptic transmission. Science 363, eaao4827 (2019).
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (grant nos. 21925402, 51802125 and 21631005). A part of this work (from N.A.K. and J.-Y.K.) was supported by NSF projects NSF 1463474, NSF 1566460 and DoD W911NF-10-1-0518.
Author information
Authors and Affiliations
Contributions
H.K., N.A.K. and C.X. conceived the project and designed the experiments. A.Q. and M.S. were responsible for cell and animal experiments. J.-Y.K. carried out the electromagnetic calculations. L.X. and C.H. carried out the assembly synthesis. W.M. and X.W. were responsible for spectroscopic measurements. H.K. and N.A.K. conceptualized the work. C.X. and H.K. supervised the study. H.K., N.A.K. and C.X. analysed the results and wrote the manuscript. X.L., H.K., N.A.K. and C.X. discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, figures, tables and references.
Rights and permissions
About this article
Cite this article
Qu, A., Sun, M., Kim, JY. et al. Stimulation of neural stem cell differentiation by circularly polarized light transduced by chiral nanoassemblies. Nat Biomed Eng 5, 103–113 (2021). https://doi.org/10.1038/s41551-020-00634-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-020-00634-4
This article is cited by
-
Regulation of biological processes by intrinsically chiral engineered materials
Nature Reviews Materials (2023)
-
Chiral nanoparticle-remodeled gut microbiota alleviates neurodegeneration via the gut–brain axis
Nature Aging (2023)
-
Circularly Polarized Light-Enabled Chiral Nanomaterials: From Fabrication to Application
Nano-Micro Letters (2023)
-
Temperature-modulated inversion and switching of chiroptical responses in dynamic side-by-side oligomers of gold nanorods
Nano Research (2023)
-
Chiral inorganic nanomaterials: Harnessing chirality-dependent interactions with living entities for biomedical applications
Nano Research (2023)