Abstract
The experimental melting data for alkali metals reported in the literature reveal that the melting temperature becomes maximum at a particular value of high pressure and then decreases with the increase in pressure. We demonstrate in the present communication that the Lindemann law does not yield negative values for the melting slopes of solids at high pressures as far as the bulk modulus increases and the Grüneisen parameter decreases with the increase in pressure such that the pressure derivative of bulk modulus and the Grüneisen parameter remain positive finite. Arafin and Singh have produced an agreement with the experimental melting data for alkali metals with the help of the Lindemann law using quadratic equations in powers of pressure for the Grüneisen parameter and bulk modulus. These quadratic equations are shown here to be inadequate and invalid at high pressures. For explaining the negative slopes of the melting curves, we need a theory of melting which should take into account the structural and electronic changes at melting. We present a discussion of some very important and highly relevant studies (Martinez-Canales and Bergara; Deng and Lee) based on fundamental considerations and ab initio calculations used recently for explaining the turnover in the melting curves of alkali metals and ferropericlase, an important Earth lower mantle mineral. The structural and electronic changes are responsible for the anomalous thermoelastic behavior yielding negative values of elastic moduli and Grüneisen parameter both, thus making the Lindemann law applicable to materials under study.




Similar content being viewed by others
References
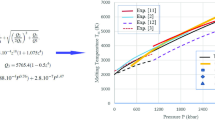
S. Arafin, R.N. Singh, Int. J. Mod. Phys. B 30, 1750031 (2016)
S. Arafin, R.N. Singh, J. Phys. Chem. Solids 91, 101–105 (2016)
F.A. Lindemann, Physik, Z. 11, 609–612 (1910)
J.J. Gilvarry, Phys. Rev. 102, 308–316 (1956)
H.D. Leudemann, G.C. Kennedy, J. Geophys. Res. 73, 2795–2805 (1968)
R. Boehler, C.S. Zha, Physica B+C 139–140, 233–236 (1986)
D. Errandonea, J. Phys. Chem. Solids 67, 2017–2026 (2006)
B. Rousseau, Y. Xie, Y. Ma, A. Bergara, Eur. Phys. J. B 81, 1–14 (2011)
J. Hama, K. Suito, J. Phys, Condens. Matter 8, 67–71 (1996)
F.D. Stacey, P.M. Davis, Phys. Earth Planet. Inter. 142, 137–184 (2004)
A. Vijay, Int. J. Mod. Phys. B 33, 1975001 (2019)
M. Martinez-Canales, A. Bergara, J. Phys. Chem. Solids 69, 2151–2154 (2008)
E. Gregoryanz, O. Degtyareva, M. Somayazulu, R.J. Hemley, H. Mao, Phys. Rev. Lett. 94, 185502 (2005)
J.B. Neaton, N.W. Ashcroft, Nature 400, 141–144 (1999)
J.B. Neaton, N.W. Ashcroft, Phys. Rev. Lett. 86, 2830–2833 (2001)
O. Narygina, E.E. MacBride, G.W. Stinton, M.I. McMahon, Phys. Rev. B 84, 054111 (2011)
L. Burakovsky, D.L. Preston, J. Phys. Chem. Solids 65, 1581–1587 (2004)
J. Shanker, B.P. Singh, H.K. Baghel, Phys. B 387, 409–414 (2007)
P.L. Dorogokupets, A.R. Oganov, Phys. Rev. B 75, 24115 (2007)
L. Knopoff, J. Geophys. Res. 68, 2929–2932 (1963)
O.L. Anderson, Equation of state of solids for Geophysics and Ceramic Science (Oxford University Press, Oxford, 1995)
F.D. Stacey, Rep. Prog. Phys. 68, 341–383 (2005)
J.C. Slater, Introduction to Chemical Physics (McGraw Hill, New York, 1939)
J.S. Dugdale, D.K.C. MacDonald, Phys. Rev. 89, 832–834 (1953)
V. YaVashchenko, V.N. Zubarev, Sov. Phys. Solid State 5, 653–655 (1963)
M.A. Barton, F.D. Stacey, Phys. Earth Planet. Inter. 39, 167–177 (1985)
K. Sunil, S.B. Sharma, B.S. Sharma, Inter. J. Mod. Phys. B 32, 1850339 (2018)
W.B. Holzapfel, M. Hartwig, W. Sievers, J. Phys. Chem. Ref. Data 30, 515–529 (2001)
J. Deng, K.K.M. Lee, Am. Miner. 104, 1189–1196 (2019)
J. Shanker, S.S. Kushwah, P. Kumar, Phys. B 239, 337–344 (1997)
S. Gaurav, B.S. Sharma, S.B. Sharma, S.C. Upadhyaya, Phys. B 332, 328–339 (2002)
S.S. Kushwah, H.C. Shrivastava, K.S. Singh, Phys. B 388, 20–25 (2007)
H.C. Shrivastava, Phys. B 404, 251–254 (2009)
S.S. Kushwah, N.K. Bhardwaj, Int. J. Mod. Phys. B 24, 1187–1200 (2010)
P.K. Vidyarthi, B.P. Singh, Phys. B 410, 259–261 (2013)
J. Shanker, P. Dulari, P.K. Singh, Phys. B 404, 4083–4085 (2009)
J. Shanker, K. Sunil, B.S. Sharma, Phys. B 407, 2082–2083 (2012)
J. Shanker, K. Sunil, B.S. Sharma, Phys. Earth Planet. Inter. 262, 41–47 (2017)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shanker, J., Anand, K., Sharma, B.S. et al. On the Applicability of Lindemann’s Law for the Melting of Alkali Metals. Int J Thermophys 41, 170 (2020). https://doi.org/10.1007/s10765-020-02751-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-020-02751-3