Seed Rain, Soil Seed Bank, and Seedling Emergence Indicate Limited Potential for Self-Recovery in a Highly Disturbed, Tropical, Mixed Deciduous Forest
Abstract
:1. Introduction
2. Results
2.1. Seed Rain: Low Seed Abundances Distributed to the Whole Study Area
2.2. Soil Seed Bank: Low Species Richness and Abundances of Tree Seed Storage
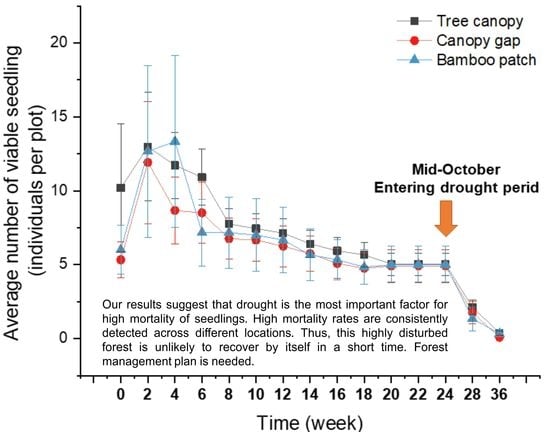
2.3. Tree Seedling Emergence and Survival Rates: Extremely Low Survival Rate in the Dry Season
2.4. Does the Combined Number of Species from the Three Methods Represent the Total Tree Species Pool at the Study Site?
3. Discussion
3.1. Low Seed Input in Both Abundance and Richness
3.2. Viable Seed Storage in Soil: Which Factors May Play an Important Role?
3.3. Low Seedling Survival Rate: There Are Many Ways to Die
3.4. Seed Rain and Seedling Community Dynamics
4. Materials and Methods
4.1. Study Area
4.2. Experimental Setup
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sanderson, E.W.; Jaiteh, M.; Levy, M.A.; Redford, K.H.; Wannebo, A.V.; Woolmer, G. The human footprint and the last of the wild. BioScience 2002, 52, 891–904. [Google Scholar] [CrossRef]
- Seppälä, R. The future of forest research in a changing world. J. For. Res. 2004, 9, 313–316. [Google Scholar] [CrossRef]
- Turner, B.L.; Lambin, E.F.; Reenberg, A. The emergence of land change science for global environmental change and sustainability. Proc. Natl. Acad. Sci. USA 2007, 104, 20666–20671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, L.; Lee, T.M.; Koh, L.P.; Brook, B.W.; Gardner, T.A.; Barlow, J.; Peres, C.A.; Bradshaw, C.J.A.; Laurance, W.F.; Lovejoy, T.E.; et al. Primary forests are irreplaceable for sustaining tropical biodiversity. Nature 2011, 478, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Purahong, W.; Kahl, T.; Schloter, M.; Bauhus, J.; Buscot, F.; Krüger, D. Comparing fungal richness and community composition in coarse woody debris in Central European beech forests under three types of management. Mycol. Prog. 2014, 13, 959–964. [Google Scholar] [CrossRef]
- Purahong, W.; Hoppe, B.; Kahl, T.; Schloter, M.; Schulze, E.-D.; Bauhus, J.; Buscot, F.; Krüger, D. Changes within a single land-use category alter microbial diversity and community structure: Molecular evidence from wood-inhabiting fungi in forest ecosystems. J. Environ. Manag. 2014, 139, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Reid, J.L.; Holl, K.D.; Zahawi, R.A. Seed dispersal limitations shift over time in tropical forest restoration. Ecol. Appl. 2015, 25, 1072–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, A.; Sato, S.; Sakai, T.; Kuramoto, S.; Tabuchi, R. A soil seed bank in a mature conifer plantation and establishment of seedlings after clear-cutting in southwest Japan. J. For. Res. 2005, 10, 295–304. [Google Scholar] [CrossRef]
- Tamura, A. Potential of soil seed banks in the ecological restoration of overgrazed floor vegetation in a cool-temperate old-growth damp forest in eastern Japan. J. For. Res. 2016, 21, 43–56. [Google Scholar] [CrossRef]
- Schafer, J.L.; Just, M.G. Size dependency of post-disturbance recovery of multi-stemmed resprouting trees. PLoS ONE 2014, 9, e105600. [Google Scholar] [CrossRef] [PubMed]
- Guariguata, M.R.; Ostertag, R. Neotropical secondary forest succession: Changes in structural and functional characteristics. For. Ecol. Manag. 2001, 148, 185–206. [Google Scholar] [CrossRef]
- Tekle, K.; Bekele, T. The role of soil seed banks in the rehabilitation of degraded hillslopes in Southern Wello, Ethiopia. Biotropica 2000, 32, 23–32. [Google Scholar] [CrossRef]
- Holl, K.D.; Loik, M.E.; Lin, E.H.V.; Samuels, I.A. Tropical montane forest restoration in Costa Rica: Overcoming barriers to dispersal and establishment. Restor. Ecol. 2000, 8, 339–349. [Google Scholar] [CrossRef]
- Chazdon, R.L. Tropical forest recovery: Legacies of human impact and natural disturbances. Perspect. Plant Ecol. Evol. Syst. 2003, 6, 51–71. [Google Scholar] [CrossRef] [Green Version]
- Cole, L.E.S.; Bhagwat, S.A.; Willis, K.J. Recovery and resilience of tropical forests after disturbance. Nat. Commun. 2014, 5, 3906. [Google Scholar] [CrossRef] [Green Version]
- Holl, K.D. Restoring tropical forest. Nat. Educ. Knowl. 2013, 4, 4. [Google Scholar]
- Chambers, J.Q.; Negron-Juarez, R.I.; Marra, D.M.; Di Vittorio, A.; Tews, J.; Roberts, D.; Ribeiro, G.H.P.M.; Trumbore, S.E.; Higuchi, N. The steady-state mosaic of disturbance and succession across an old-growth Central Amazon forest landscape. Proc. Natl. Acad. Sci. USA 2013, 110, 3949–3954. [Google Scholar] [CrossRef] [Green Version]
- Moles, A.T.; Drake, D.R. Potential contributions of the seed rain and seed bank to regeneration of native forest under plantation pine in New Zealand. N. Z. J. Bot. 1999, 37, 83–93. [Google Scholar] [CrossRef]
- Catovsky, S.; Bazzaz, F.A. The role of resource interactions and seedling regeneration in maintaining a positive feedback in hemlock stands. J. Ecol. 2000, 88, 100–112. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Guo, Q.; Gao, X.; Ma, K. Seed rain, soil seed bank, seed loss and regeneration of Castanopsis fargesii (Fagaceae) in a subtropical evergreen broad-leaved forest. For. Ecol. Manag. 2007, 238, 212–219. [Google Scholar] [CrossRef]
- Ceccon, E.; Hernández, P. Seed rain dynamics following disturbance exclusion in a secondary tropical dry forest in Morelos, Mexico. Rev. Biol. Trop. 2009, 57, 257–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grombone-Guaratini, M.T.; Alves, L.F.; Vinha, D.; Franco, G.A.D.C. Seed rain in areas with and without bamboo dominance within an urban fragment of the Atlantic Forest. Acta Bot. Bras. 2014, 28, 76–85. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Ramos, M.; Soto-Castro, A. Seed rain and advanced regeneration in a tropical rain forest. Vegetatio 1993, 107, 299–318. [Google Scholar] [CrossRef]
- Dhillion, S.; Ampornpan, L.; Austreng, I. Land Use and Plant Diversity in Ban Bung and Na Haeo Forest Reserve; Craftman Press: Bangkok, Thailand, 2003. [Google Scholar]
- Larpkern, P.; Moe, S.R.; Totland, Ø. Bamboo dominance reduces tree regeneration in a disturbed tropical forest. Oecologia 2011, 165, 161–168. [Google Scholar] [CrossRef]
- Sakai, S.; Momose, K.; Yumoto, T.; Nagamitsu, T.; Nagamasu, H.; Hamid, A.A.; Nakashizuka, T. Plant reproductive phenology over four years including an episode of general flowering in a lowland dipterocarp forest, Sarawak, Malaysia. Am. J. Bot. 1999, 86, 1414–1436. [Google Scholar] [CrossRef] [Green Version]
- Vázquez-Yanes, C.; Orozco-Segovia, A. Patterns of seed longevity and germination in the tropical rainforest. Annu. Rev. Ecol. Syst. 1993, 24, 69–87. [Google Scholar] [CrossRef]
- Rother, D.C.; Rodrigues, R.R.; Pizo, M.A. Effects of bamboo stands on seed rain and seed limitation in a rainforest. For. Ecol. Manag. 2009, 257, 885–892. [Google Scholar] [CrossRef]
- Olano, J.M.; Caballero, I.; Escudero, A. Soil seed bank recovery occurs more rapidly than expected in semi-arid Mediterranean gypsum vegetation. Ann. Bot. 2012, 109, 299–307. [Google Scholar] [CrossRef] [Green Version]
- Gallery, R.E.; Moore, D.J.P.; Dalling, J.W. Interspecific variation in susceptibility to fungal pathogens in seeds of 10 tree species in the neotropical genus Cecropia. J. Ecol. 2010, 98, 147–155. [Google Scholar] [CrossRef]
- Salinas-Peba, L.; Parra-Tabla, V.; Campo, J.; Munguía-Rosas, M.A. Survival and growth of dominant tree seedlings in seasonally tropical dry forests of Yucatan: Site and fertilization effects. J. Plant Ecol. 2013. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, A.W.; Medina-Vega, J.A.; Malhi, Y.; Adu-Bredu, S.; Ametsitsi, G.K.D.; Djagbletey, G.; van Langevelde, F.; Veenendaal, E.; Oliveras, I. Winners and losers: Tropical forest tree seedling survival across a West African forest–savanna transition. Ecol. Evol. 2016, 6, 3417–3429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engelbrecht, B.M.J.; Kursar, T.A.; Tyree, M.T. Drought effects on seedling survival in a tropical moist forest. Trees 2005, 19, 312–321. [Google Scholar] [CrossRef]
- Norden, N.; Chave, J.; Caubère, A.; Châtelet, P.; Ferroni, N.; Forget, P.-M.; Thébaud, C. Is temporal variation of seedling communities determined by environment or by seed arrival? A test in a neotropical forest. J. Ecol. 2007, 95, 507–516. [Google Scholar] [CrossRef]
- Ceccon, E.; Huante, P.; Rincón, E. Abiotic factors influencing tropical dry forests regeneration. Braz. Arch. Biol. Technol. 2006, 49, 305–312. [Google Scholar] [CrossRef]
- Singhakumara, B.M.P.; Uduporuwa, R.S.J.P.; Ashton, P.M.S. Soil seed banks in relation to light and topographic position of a hill dipterocarp forest in Sri Lanka1. Biotropica 2000, 32, 190–196. [Google Scholar] [CrossRef]
- Butler, B.J.; Chazdon, R.L. Species richness, spatial variation, and abundance of the soil seed bank of a secondary tropical rain forest1. Biotropica 1998, 30, 214–222. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Waiboonya, P.; Elliott, S. Sowing time and direct seeding success of native tree species for restoring tropical forest ecosystems in northern Thailand. New For. 2020, 51, 81–99. [Google Scholar] [CrossRef]
- Marks, D. Climate change and Thailand: Impact and response. Contemp. Southeast Asia 2011, 33, 229–258. [Google Scholar] [CrossRef]






| Tree Species | Seed Rain | Seed Bank (1) | Seed Bank (2) | Seedling Emergence |
|---|---|---|---|---|
| Aporosa octandra (B.-H. ex D.Don) Vick. var. octandra | - | - | - | 2 |
| Aporosa octandra (B.-H. ex D.Don) Vick. var. yunnanensis | - | - | - | 8 |
| Bauhinia malabarica Roxb. | 165 | - | - | - |
| Bombax anceps Pierre var. anceps | - | - | - | 2 |
| Canarium subulatum Guill. | 86 | 7 | 62 | 214 |
| Cratoxylum formosum (Jack) Dyer | 5 | - | - | 26 |
| Croton roxburghii N.P. Balakr. | 6 | 1 | - | 35 |
| Dalbergia sp. | 1 | - | - | - |
| Gardenia sootepensis Hutch. | 549 | 7 | - | 210 |
| Garuga pinnata Roxb. | 43 | - | - | 2 |
| Irvingia malayana Oliv. ex A.W.Benn | 3 | - | 4 | - |
| Lagerstroemia (aff. venusta Wall. ex Cl.) | 120 | 15 | - | 188 |
| Memecylon edule Roxb. | 17 | - | - | 24 |
| Microcos tomentosa Sm. | - | - | - | 2 |
| Pterocarpus macrocarpus Kurz | 58 | - | - | 4 |
| Shorea roxburghii G.Don | 1 | - | - | - |
| Suregada multiflora (A. Juss.) Baill. | - | - | - | 6 |
| Terminalia chebula Retz. var. chebula | - | - | - | 1 |
| Terminalia triptera Stapf. | 2 | - | - | 3 |
| Sum | 1056 | 30 | 66 | 727 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalermsri, A.; Ampornpan, L.-a.; Purahong, W. Seed Rain, Soil Seed Bank, and Seedling Emergence Indicate Limited Potential for Self-Recovery in a Highly Disturbed, Tropical, Mixed Deciduous Forest. Plants 2020, 9, 1391. https://doi.org/10.3390/plants9101391
Chalermsri A, Ampornpan L-a, Purahong W. Seed Rain, Soil Seed Bank, and Seedling Emergence Indicate Limited Potential for Self-Recovery in a Highly Disturbed, Tropical, Mixed Deciduous Forest. Plants. 2020; 9(10):1391. https://doi.org/10.3390/plants9101391
Chicago/Turabian StyleChalermsri, Anussara, La-aw Ampornpan, and Witoon Purahong. 2020. "Seed Rain, Soil Seed Bank, and Seedling Emergence Indicate Limited Potential for Self-Recovery in a Highly Disturbed, Tropical, Mixed Deciduous Forest" Plants 9, no. 10: 1391. https://doi.org/10.3390/plants9101391