Antioxidant Responses of Phenolic Compounds and Immobilization of Copper in Imperata cylindrica, a Plant with Potential Use for Bioremediation of Cu Contaminated Environments
Abstract
:1. Introduction
2. Results
2.1. Biomass Production
2.2. Lipid Peroxidation
2.3. Cu Concentration in Plant and Cu Available in Substrate
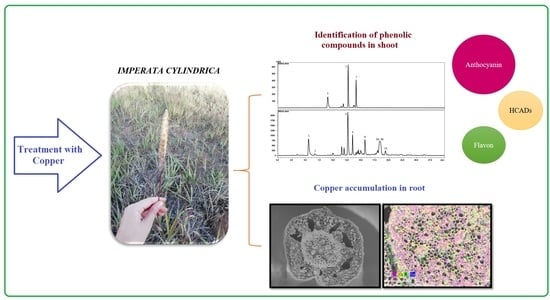
2.4. Quantification and Identification of Phenolic Compounds in Shoot
2.5. Total Phenols in Shoot and Antioxidant Activity
2.6. Multivariate Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Collection and Experimental Design
4.2. Lipid Peroxidation
4.3. Cu Concentration
4.4. Cu Localization in Plant Tissues
4.5. Identification and Quantification of Phenolic Compounds
4.6. Total Phenols and Antioxidant Activity Determinations
4.7. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Choppala, G.K.; Bolan, N.S.; Megharaj, M.; Chen, Z.; Naidu, R. The Influence of Biochar and Black Carbon on Reduction and Bioavailability of Chromate in Soils. J. Environ. Qual. 2012, 41, 1175. [Google Scholar] [CrossRef]
- Moore, F.; González, M.E.; Khan, N.; Curaqueo, G.; Sanchez-Monedero, M.; Rilling, J.; Morales, E.; Panichini, M.; Mutis, A.; Jorquera, M.; et al. Copper immobilization by biochar and microbial community abundance in metal-contaminated soils. Sci. Total Environ. 2018, 616–617, 960–969. [Google Scholar] [CrossRef]
- Ortiz, J.; Soto, J.; Almonacid, L.; Fuentes, A.; Arriagada, C. Alleviation of metal stress by Pseudomonas orientalis and Chaetomium cupreum strains and their effects on Eucalyptus globulus growth promotion. Plant Soil 2019, 436, 449–461. [Google Scholar] [CrossRef]
- Berni, R.; Luyckx, M.; Xu, X.; Legay, S.; Sergeant, K. Reactive oxygen species and heavy metal stress in plants: Impact on the cell wall and secondary metabolism. Environ. Exp. Bot. 2019, 161, 98–106. [Google Scholar] [CrossRef]
- El-Meihy, R.M.; Abou-aly, H.E.; Youssef, A.M.; Tewfike, T.A.; El-Alkshar, E.A. Efficiency of heavy metals-tolerant plant growth promoting bacteria for alleviating heavy metals toxicity on sorghum. Environ. Exp. Bot. 2019, 162, 295–301. [Google Scholar] [CrossRef]
- Jones, A.; Huang, J.-H.; Borrelli, P.; Orgiazzi, A.; Ballabio, C.; Lugato, E.; Fernández-Ugalde, O.; Montanarella, L.; Panagos, P. Copper distribution in European topsoils: An assessment based on LUCAS soil survey. Sci. Total Environ. 2018, 636, 282–298. [Google Scholar] [CrossRef]
- Ravet, K.; Pilon, M. Copper and Iron Homeostasis in Plants: The Challenges of Oxidative Stress. Antioxid. Redox Signal. 2012, 19, 919–932. [Google Scholar] [CrossRef] [Green Version]
- Cornejo, P.; Meier, S.; Borie, G.; Rillig, M.C.; Borie, F. Glomalin-related soil protein in a Mediterranean ecosystem affected by a copper smelter and its contribution to Cu and Zn sequestration. Sci. Total Environ. 2008, 406, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Ginocchio, R. Effects of a copper smelter on a grassland community in the Puchuncaví Valley, Chile. Chemosphere 2000, 41, 15–23. [Google Scholar] [CrossRef]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M.; Antonio, C. Heavy Metal Tolerance in Plants: Role of Transcriptomics. Proteomics 2016, 6, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Selmar, D.; Kleinwächter, M.; Abouzeid, S.; Yahyazadeh, M.; Nowak, M. The Impact of Drought Stress on the Quality of Spice and Medicinal Plants. In Medicinal Plants and Environmental Challenges; Ghorbanpour, M., Varma, A., Eds.; Springer: Cham, Switzerland, 2017; pp. 159–175. [Google Scholar] [CrossRef]
- Kisa, D.; Elmastas, M.; Ozturk, L.; Kayir, O. Responses of the phenolic compounds of Zea mays under heavy metal stress. App. Biol. Chem. 2016, 59, 813–820. [Google Scholar] [CrossRef]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Mellen, P.B.; Walsh, T.F.; Herrington, D.M. Whole grain intake and cardiovascular disease: A meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Sreeramulu, D.; Reddy, C.V.; Raghunath, M. Antioxidant activity of commonly consumed cereals, millets, pulses and legumes in India. Indian J. Biochem. Biophys. 2009, 46, 112–115. [Google Scholar] [PubMed]
- Manquián-Cerda, K.; Cruces, E.; Escudey, M.; Zúñiga, G.; Calderón, R. Interactive effects of aluminum and cadmium on phenolic compounds, antioxidant enzyme activity and oxidative stress in blueberry (Vaccinium corymbosum L.) plantlets cultivated in vitro. Ecotoxicol. Environ. Saf. 2018, 150, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Fuente, V.; Rufo, L.; Juárez, B.H.; Menéndez, N.; García-Hernández, M.; Salas-Colera, E.; Espinosa, A. Formation of biomineral iron oxides compounds in a Fe hyperaccumulator plant: Imperata cylindrica (L.) P. Beauv. J. Struct. Biol. 2016, 193, 23–32. [Google Scholar] [CrossRef]
- Amils, R.; Rodriguez, N.; de la Fuente, V. Composition, speciation and distribution of iron minerals in Imperata cylindrica. Plant Physiol. Biochem. 2007, 20, 1–6. [Google Scholar] [CrossRef]
- Rodríguez, N.; Menéndez, N.; Tornero, J.; Amils, R.; De La Fuente, V. Internal iron biomineralization in Imperata cylindrica, a perennial grass: Chemical composition, speciation and plant localization. New Phytol. 2005, 165, 781–789. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, J.Z. Identification and Growth Inhibitory Activity of the Chemical Constituents from Imperata Cylindrica Aerial Part Ethyl Acetate Extract. Molecules 2018, 23, 1807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meier, S.; Alvear, M.; Aguilera, P.; Ginocchio, R.; Borie, F.; Cornejo, P. Influence of copper on root exudate patterns in some metallophytes and agricultural plants. Ecotoxicol. Environ. Saf. 2012, 75, 8–15. [Google Scholar] [CrossRef]
- Tsay, L.; Wang, W.; Chen, Y. Plant response to Cu toxicity. Taiwana 1995, 40, 173–181. [Google Scholar]
- Potters, G.; Pasternak, T.P.; Guisez, Y.; Palme, K.J.; Jansen, M.A.K. Stress-induced morphogenic responses: Growing out of trouble? Trends Plant Sci. 2007, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, P.R.; Ambrosini, V.G.; Miotto, A.; Ceretta, C.A.; Simão, D.G.; Brunetto, G. Black Oat (Avena strigosa Schreb.) Growth and Root Anatomical Changes in Sandy Soil with Different Copper and Phosphorus Concentrations. Water Air Soil Pollut. 2016, 227, 192. [Google Scholar] [CrossRef]
- Montiel-Rozas, M.M.; Madejón, E.; Madejón, P. Effect of heavy metals and organic matter on root exudates (low molecular weight organic acids) of herbaceous species: An assessment in sand and soil conditions under different levels of contamination. Environ. Pollut. 2016, 216, 273–281. [Google Scholar] [CrossRef] [Green Version]
- De Conti, L.; Ceretta, Q.A.; Tiecher, T.L.; Silva, L.O.; Tassinari, A.; Somavilla, L.M.; Mimmo, T.; Cesco, S.; Brunetto, G. Growth and chemical changes in the rhizosphere of black oat (Avena strigosa) grown in soils contaminated with copper. Ecotoxicol. Environ. Saf. 2018, 163, 19–27. [Google Scholar] [CrossRef]
- Manara, A. Plant Responses to Heavy Metal Toxicity. In Plants and Heavy Metals; Furini, A., Ed.; Springer Briefs in Molecular Science; Springer: Dordrecht, The Netherlands, 2012; pp. 27–54. [Google Scholar] [CrossRef]
- Yin, L.; Hasan, M.K.; Shi, K.; Zhou, Y.; Ahammed, G.J.; Zhou, J.; Xia, X.; Yu, J. Melatonin mitigates cadmium phytotoxicity through modulation of phytochelatins biosynthesis, vacuolar sequestration, and antioxidant potential in Solanum lycopersicum L. Front. Plant Sci. 2015, 6, 1–14. [Google Scholar] [CrossRef]
- Zhao, Y.; Chengcai, C. Towards understanding plant response to heavy metal stress. In Abiotic Stress in Plants—Mechanisms and Adaptations; Shanker, A., Ed.; Tech Europe: Rijeka, Croatia, 2011; pp. 59–78. [Google Scholar] [CrossRef] [Green Version]
- González, I.; Muena, V.; Cisternas, M.; Neaman, A. Copper accumulation in a plant community affected by mining contamination in Puchuncaví valley, central Chile. Rev. Chil. Hist. Nat. 2008, 81, 279–291. [Google Scholar]
- Nada, E.; Ferjani, B.A.; Ali, R.; Bechir, B.R.; Imed, M.; Makki, B. Cadmium-induced growth inhibition and alteration of biochemical parameters in almond seedlings grown in solution culture. Acta Physiol. Plant. 2006, 29, 57–62. [Google Scholar] [CrossRef]
- Cornejo, P.; Meier, S.; García, S.; Borie, F.; Seguel, A.; Ferrol, N.; Durán, P. Contribution of inoculation with arbuscular mycorrhizal fungi to the bioremediation of a copper polluted soil using Oenothera picensis. J. Soil Sci. Plant Nutr. 2017, 17, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Meier, S.; Azcón, R.; Cartes, P.; Borie, F.; Cornejo, P. Alleviation of Cu toxicity in Oenothera picensis by copper-adapted arbuscular mycorrhizal fungi and treated agrowaste residue. Appl. Soil Ecol. 2011, 48, 117–124. [Google Scholar] [CrossRef]
- Meier, S.; Curaqueo, G.; Khan, N.; Bolan, N.; Rilling, J.; Vidal, C.; Fernández, N.; Acuña, J.; González, M.-E.; Cornejo, P.; et al. Effects of biochar on copper immobilization and soil microbial communities in a metal-contaminated soil. J. Soils Sediments 2015, 17, 1237–1250. [Google Scholar] [CrossRef]
- Meier, S.; Borie, F.; Curaqueo, G.; Bolan, N.; Cornejo, P. Effects of arbuscular mycorrhizal inoculation on metallophyte and agricultural plants growing at increasing copper levels. Appl. Soil Ecol. 2012, 61, 280–287. [Google Scholar] [CrossRef]
- Chen, Y.A.; Chi, W.C.; Huang, T.L.; Lin, C.Y.; Quynh Nguyeh, T.T.; Hsiung, Y.C.; Chia, L.C.; Huang, H.J. Mercury-induced biochemical and proteomic changes in rice roots. Plant Physiol. Biochem. 2012, 55, 23–32. [Google Scholar] [CrossRef]
- Pandey, N.; Pathak, G.C.; Pandey, D.K.; Pandey, R. Heavy metals, Co, Ni, Cu, Zn and Cd, produce oxidative damage and evoke differential antioxidant responses in spinach. Brazilian J. Plant Physiol. 2009, 21, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Xuan, L.I.; Bin-feng, Z.; Li, Y.; Gui-xin, C.; Zheng-tao, W. Two new chromones and a new flavone glycoside from Imperata cylindrica. Chin. J. Nat. Med. 2013, 11, 77–80. [Google Scholar] [CrossRef]
- Ruiz, A.; Sanhueza, M.; Gómez, F.; Tereucán, G.; Valenzuela, T.; García, S.; Cornejo, P.; Hermosín-Gutiérrez, I. Changes in the content of anthocyanins, flavonols, and antioxidant activity in Fragaria ananassa var. Camarosa fruits under traditional and organic fertilization. J. Sci. Food Agric. 2018, 99, 2404–2410. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Qiao, X.; Bo, T.; Wang, Q.; Guo, D.; Ye, M. Low energy induced homolytic fragmentation of flavonol 3- O-glycosides by negative electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2014, 28, 385–395. [Google Scholar] [CrossRef]
- Costa, W.; Cardoso, E.; de Melo, W.; de Barros, W.M.; Converti, A.; Bragagnolo, N. Design and evaluation of microencapsulated systems containing extract of whole green coffee fruit rich in phenolic acids. Food Hydrocoll. 2020, 100, 1–8. [Google Scholar] [CrossRef]
- Lee, T.; Shih, T.; Huang, M.; Lin, K.; Huang, W.; Yang, C. Eliminating interference by anthocyanins when determining the porphyrin ratio of red plant leaves. J. Photochem. Photobiol. B Biol. 2018, 187, 106–112. [Google Scholar] [CrossRef]
- Aguilera, A.; Tereucán, G.; Ercoli, S.; Cornejo, P.; Gomez, R.; Uhlmann, L.; Guigas, C. Influence of Organic and Chemical Fertilization on Antioxidant Compounds Profiles and Activities in Fruits of Fragaria ananassa var. Camarosa. J Soil Sci. Plant Nutr. 2020. [Google Scholar] [CrossRef]
- Steyn, W.J.; Wand, S.J.; Holcroft, D.M.; Jacobs, G. Anthocyanins in vegetative tissues: A proposed unified function in photoprotection. New Phytol. 2002, 155, 349–361. [Google Scholar] [CrossRef]
- Cambrollé, J.; García, J.L.; Ocete, R.; Figueroa, M.E.; Cantos, M. Growth and photosynthetic responses to copper in wild grapevine. Chemosphere 2013, 93, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Cambrollé, J.; García, J.L.; Figueroa, M.E.; Cantos, M. Evaluating wild grapevine tolerance to copper toxicity. Chemosphere 2015, 120, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Sruthi, P.; Puthur, J.T. Characterization of physiochemical and anatomical features associated with enhanced phytostabilization of copper in Bruguiera cylindrica (L.) Blume. Int. J. Phytoremediat. 2019, 21, 1423–1441. [Google Scholar] [CrossRef]
- Aponte, H.; Herrera, W.; Cameron, C.; Black, H.; Meier, S.; Paolini, J.; Tapia, Y.; Cornejo, P. Alteration of enzyme activities and functional diversity of a soil contaminated with copper and arsenic. Ecotox. Environ. Saf. 2020, 192, 110264. [Google Scholar] [CrossRef]
- Du, Z.; Bramlage, W.J. Modified Thiobarbituric Acid Assay for Measuring Lipid Oxidation in Sugar-Rich Plant Tissue Extracts. J. Agric. Food Chem. 1992, 40, 1566–1570. [Google Scholar] [CrossRef]
- Santander, C.; Ruiz, A.; García, S.; Aroca, R.; Cumming, J.; Cornejo, P. Efficiency of two arbuscular mycorrhizal fungal inocula to improve saline stress tolerance in lettuce plants by changes of antioxidant defense mechanisms. J. Sci. Food Agric. 2020, 100, 1577–1587. [Google Scholar] [CrossRef]
- Parada, J.; Valenzuela, T.; Gómez, F.; Tereucán, G.; García, S.; Cornejo, P.; Ruiz, A. Effect of fertilization and arbuscular mycorrhizal fungal inoculation on antioxidant profiles and activities in Fragaria ananassa fruit. J. Sci. Food Agric. 2019, 99, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.; Vitic, A.J. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]





| Peak | tR (min) | Group | Compound | ʎ max | [M + H]+ | [M − H]− | Productions |
|---|---|---|---|---|---|---|---|
| 1 | 5.5 | HCADs | 5-caffeoylquinic acid | 324 | 353.5 | 191.2 | |
| 2 | 6.6 | HCADs | caffeoylquinic acid isomer | 323 | 353.6 | 191.2 | |
| 3 | 8.8 | Anthocyanin | cyanidin-3-hexoside | 515 | 449.8 | 287.5 | |
| 4 | 12.5 | Flavon | Orientin | 348 | 447.6 | 357.3; 327.3; 430.3; 297.4; 287.3 | |
| 5 | 12.6 | Anthocyanin | cyanidin-3-malonylglucoside | 516 | 535.7 | 287.2 | |
| 6 | 13.4 | * Flavonoid glycosides | * | 348 | 549.7 | 400.3; 370.4; 460.4; 490.4; 430.3 | |
| 7 | 14.0 | Anthocyanin | cyanidin-derivative | 517 | 621.8 | 287.5 | |
| 8 | 15.7 | * Flavonoid glycosides | * | 345 | 417.6 | 358.3; 328.2; 400.3; 298.3 | |
| 9A | 18.1 | * Flavonoid glycosides | * | 348 | 575.7 | 326.2; 298.3; 412.3; 430.3 | |
| 9B | 18.3 | * Flavonoid glycosides | * | 349 | 575.7 | 412.3; 474.3; 298.3; 338.2; 309.2 | |
| 10 | 19.3 | * Flavonoid glycosides | * | 346 | 431.6 | 358.3; 328.1; 298.2; 285.2 |
| N Compound | Phenolic Compound | µg g−1 FW | |
|---|---|---|---|
| Control | Treatment | ||
| 1 | 5-caffeoylquinic acid | 1387.1 ± 107.1 b | 1715.0 ± 136.3 a |
| 2 | caffeoylquinic acid isomer | 217.6 ± 9.3 a | 247.1 ± 45.4 a |
| 3 | cyanidin-3-hexoside | 13.3 ± 2.3 b | 33.3 ± 7.3 a |
| 4 | Orientin | 242.6 ± 39.3 b | 395.5 ± 78.8 a |
| 5 | cyanidin-3-malonylglucoside | 30.9 ± 4.0 b | 72.3 ± 13.4 a |
| 6 | * | 113.7 ± 24.1 a | 142.7 ± 18.1 a |
| 7 | cyanidin-derivative | 24.5 ± 4.0 b | 46.1 ± 8.7 a |
| 8 | * | 101.1 ± 23.9 b | 146.2 ± 25.9 a |
| 9A and 9B | * | 391.9 ± 7.8 a | 389.8 ± 76.5 a |
| 10 | * | 60.7 ± 11.1 a | 71.2 ± 10.4 a |
| Total phenols | 2583.4 ± 0.02 b | 3259.2 ± 0.05 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidal, C.; Ruiz, A.; Ortiz, J.; Larama, G.; Perez, R.; Santander, C.; Ferreira, P.A.A.; Cornejo, P. Antioxidant Responses of Phenolic Compounds and Immobilization of Copper in Imperata cylindrica, a Plant with Potential Use for Bioremediation of Cu Contaminated Environments. Plants 2020, 9, 1397. https://doi.org/10.3390/plants9101397
Vidal C, Ruiz A, Ortiz J, Larama G, Perez R, Santander C, Ferreira PAA, Cornejo P. Antioxidant Responses of Phenolic Compounds and Immobilization of Copper in Imperata cylindrica, a Plant with Potential Use for Bioremediation of Cu Contaminated Environments. Plants. 2020; 9(10):1397. https://doi.org/10.3390/plants9101397
Chicago/Turabian StyleVidal, Catalina, Antonieta Ruiz, Javier Ortiz, Giovanni Larama, Rodrigo Perez, Christian Santander, Paulo Ademar Avelar Ferreira, and Pablo Cornejo. 2020. "Antioxidant Responses of Phenolic Compounds and Immobilization of Copper in Imperata cylindrica, a Plant with Potential Use for Bioremediation of Cu Contaminated Environments" Plants 9, no. 10: 1397. https://doi.org/10.3390/plants9101397