Abstract
Purpose
The aim of this work was to identify whether biochemical and physiological sources of mAb pharmacokinetic sex-effects could be identified in the rat model where target-mediated disposition is avoided.
Methods
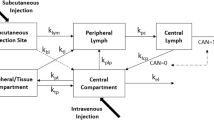
Plasma and lymphatic pharmacokinetics of the humanised anti-EGFR antibody cetuximab, along with potential physiological and biochemical drivers of pharmacokinetic sex differences, were examined in male and female rats. Cetuximab was used as a model mAb since plasma clearance is slower in female patients.
Results
When plasma concentrations were normalised to dose, female rats displayed slower plasma clearance than males, but no significant differences were observed in liver and spleen biodistribution. Sex differences in apparent plasma clearance, however, were abolished after normalisation to body weight, surface area or fat-free mass. Significant sex differences were observed in plasma testosterone, endogenous IgG and fat free mass, but did not correlate with apparent clearance. Females did, however, show two-fold higher lymphatic exposure compared to males.
Conclusions
These data suggested that mAbs more efficiently access lymph in females, but this does not affect plasma pharmacokinetics or biodistribution. Further, the data suggest that sex differences observed in humans could be a function of antigen density.






Similar content being viewed by others
Abbreviations
- EGFR:
-
Epidermal growth factor receptor
- FcRn:
-
Neonatal Fc receptor
- FcγR:
-
Fc gamma receptor
- FFM:
-
Fat free mass
- mAb:
-
Monoclonal antibody
- MPS:
-
Mononuclear phagocyte system
- TMDD:
-
Target mediated drug disposition
- WCC:
-
Peripheral white blood cell count
References
Abdiche YN, Yeung YA, Chaparro-Riggers J, Barman I, Strop P, Chin SM, et al. The neonatal fc receptor (FcRn) binds independently to both sites of the IgG homodimer with identical affinity. MAbs. 2015;7(2):331–43.
André F, Ciccolini J, Spano J-P, Penault-Llorca F, Mounier N, Freyer G, et al. Personalized medicine in oncology: where have we come from and where are we going? Pharmacogenomics. 2013;14(8):931–9.
Bird MD, Karavitis J, Kovacs EJ. Sex differences and estrogen modulation of the cellular immune response after injury. Cell Immunol. 2008;252(1–2):57–67.
Boer P. Estimated lean body mass as an index for normalization of body fluid volumes in humans. Am J Physiol-Renal Physiol. 1984;247(4):F632–6.
Centre for Drug Evaluation and Research. Clinical pharmacology and biopharmaceutics review(s): Erbitux STN/BLA 125084. 2004: 70.
Chan LJ, Bulitta JB, Ascher DB, Haynes JM, McLeod VM, Porter CJ, et al. PEGylation does not significantly change the initial intravenous or subcutaneous pharmacokinetics or lymphatic exposure of trastuzumab in rats but increases plasma clearance after subcutaneous administration. Mol Pharm. 2015;12(3):794–809.
Chan LJ, Ascher DB, Yadav R, Bulitta JB, Williams CC, Porter CJH, et al. Conjugation of 10 kDa linear PEG onto trastuzumab fab’ is sufficient to significantly enhance lymphatic exposure while preserving in vitro biological activity. Mol Pharm. 2016;13(4):1229–41.
Chan LJ, Ascher DB, Yadav R, Bulitta JB, Williams CC, Porter CJ, et al. Conjugation of 10 kDa linear PEG onto trastuzumab Fab’ is sufficient to significantly enhance lymphatic exposure while preserving in vitro biological activity. Mol Pharmacol. 2016;13(4):1229–41.
Chao T-C, Phuangsab A, Van Alten PJ, Walter RJ. Steroid sex hormones and macrophage function: regulation of chemiluminescence and phagocytosis. Am J Reprod Immunol. 1996;35(2):106–13.
Dahlberg AM, Kaminskas LM, Smith A, Nicolazzo JA, Porter CJH, Bulitta JB, et al. The lymphatic system plays a major role in the intravenous and subcutaneous pharmacokinetics of trastuzumab in rats. Mol Pharm. 2014;11(2):496–504.
Datta-Mannan A, Lu J, Witcher DR, Leung D, Tang Y, Wroblewski VJ. The interplay of non-specific binding, target-mediated clearance and FcRn interactions on the pharmacokinetics of humanized antibodies. MAbs. 2015;7(6):1084–93.
Dirks NL, Nolting A, Kovar A, Meibohm B. Population pharmacokinetics of cetuximab in patients with squamous cell carcinoma of the head and neck. J Clin Pharmacol. 2008;48(3):267–78.
EMEA. Erbitux-H-C-558-II-0005: EPAR – scientific discussion. 2004:47.
Ezan E, Becher F, Fenaille F. Assessment of the metabolism of therapeutic proteins and antibodies. Expert Opin Drug Metab Toxicol. 2014;10(8):1079–91.
Fakih M, Vincent M. Adverse events associated with anti-EGFR therapies for the treatment of metastatic colorectal cancer. Curr Oncol. 2010;17(Suppl 1):S18–30.
Fasanmade AA, Adedokun OJ, Ford J, Hernandez D, Johanns J, Hu C, et al. Population pharmacokinetic analysis of infliximab in patients with ulcerative colitis. Eur J Clin Pharmacol. 2009;65(12):1211–28.
Gouma E, Simos Y, Verginadis I, Lykoudis E, Evangelou A, Karkabounas S. A simple procedure for estimation of total body surface area and determination of a new value of Meeh’s constant in rats. Lab Anim. 2012;46(1):40–5.
Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJT. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov. 2010;9:325–38.
He X, Cruz JL, Joseph S, Pett N, Chew HY, Tuong ZK, et al. Characterization of 7A7, an anti-mouse EGFR monoclonal antibody proposed to be the mouse equivalent of cetuximab. Oncotarget. 2018;9(15):12250–60.
Hendrikx JJMA, Haanen JBAG, Voest EE, Schellens JHM, Huitema ADR, Beijnen JH. Fixed dosing of monoclonal antibodies in oncology. Oncologist. 2017;22(10):1212–21.
Hoeben BAW, Molkenboer-Kuenen JDM, Oyen WJG, Peeters WJM, Kaanders JHAM, Bussink J, et al. Radiolabeled cetuximab: dose optimization for epidermal growth factor receptor imaging in a head-and-neck squamous cell carcinoma model. Int J Cancer. 2011;129(4):870–8.
Jaber JJ, Zender CA, Mehta V, Davis K, Ferris RL, Lavertu P, et al. A multi-institutional investigation of the prognostic value of lymph nodal yield in advanced stage oral cavity squamous cell carcinoma (OCSCC). Head Neck. 2014;36(10):1446–52.
Jatoi A, Green EM, Rowland KM Jr, Sargent DJ, Alberts SR. Clinical predictors of severe cetuximab-induced rash: observations from 933 patients enrolled in north central cancer treatment group study N0147. Oncology. 2009;77(2):120–3.
Kaminskas LM, Wu Z, Barlow N, Krippner GY, Boyd BJ, Porter CJ. Partly-PEGylated poly-L-lysine dendrimers have reduced plasma stability and circulation times compared with fully PEGylated dendrimers. J Pharm Sci. 2009;98(10):3871–5.
Kaminskas LM, Kota J, McLeod VM, Kelly BD, Karellas P, Porter CJ. PEGylation of polylysine dendrimers improves absorption and lymphatic targeting following SC administration in rats. J Control Release. 2009;140(2):108–16.
Kaminskas LM, Ascher DB, McLeod VM, Herold MJ, Le CP, Sloan EK, et al. PEGylation of interferon α2 improves lymphatic exposure after subcutaneous and intravenous administration and improves antitumour efficacy against lymphatic breast cancer metastases. J Control Release. 2013;168(2):200–8.
Kaminskas LM, McLeod VM, Ascher DB, Ryan GM, Jones S, Haynes JM, et al. Methotrexate-conjugated PEGylated dendrimers show differential patterns of deposition and activity in tumor-burdened lymph nodes after intravenous and subcutaneous administration in rats. Mol Pharm. 2015;12(2):432–43.
Kwon S, Agollah GD, Wu G, Sevick-Muraca EM. Spatio-temporal changes of lymphatic contractility and drainage patterns following lymphadenectomy in mice. PLoS One. 2014;9(8):e106034.
Lindenberger M, Länne T. Sex-related effects on venous compliance and capillary filtration in the lower limb. Am J Phys Regul Integr Comp Phys. 2007;292(2):R852–9.
Liu L. Pharmacokinetics of monoclonal antibodies and fc-fusion proteins. Protein Cell. 2018;9(1):15–32.
Lu J-F, Bruno R, Eppler S, Novotny W, Lum B, Gaudreault J. Clinical pharmacokinetics of Bevacizumab in patients with solid tumors. Cancer Chemother Pharmacol. 2008;62(5):779–86.
Ma P, Yang BB, Wang YM, Peterson M, Narayanan A, Sutjandra L, et al. Population pharmacokinetic analysis of panitumumab in patients with advanced solid tumors. J Clin Pharmacol. 2009;49(10):1142–56.
Mould DR, Sweeney KRD. The pharmacokinetics and pharmacodynamics of monoclonal antibodies: mechanistic modeling applied to drug development. Curr Opin Drug Discov Dev. 2007;10(1):84–96.
Öztas B, Ćmurcu S, Kaya M. Influence of sex on the blood brain barrier permeability during bicuculline-induced seizures. Int J Neurosci. 1992;65(1–4):131–9.
Pyzik M, Rath T, Lencer WI, Baker K, Blumberg RS. FcRn: the architect behind the immune and non-immune functions of IgG and albumin. J Immunol (Baltimore, Md: 1950). 2015;194(10):4595–603.
Ryman JT, Meibohm B. Pharmacokinetics of monoclonal antibodies. CPT Pharmacometrics Syst Pharmacol. 2017;6(9):576–88.
Schmetzer O, Flörcken A. Sex differences in the drug therapy for oncologic diseases. In: Regitz-Zagrosek V, editor. Sex and gender differences in pharmacology. Berlin: Springer; 2012. p. 411–42.
Seitz K, Zhou G. Pharmacokinetic drug-drug interaction potentials for therapeutic monoclonal antibodies: reality check. J Clin Pharmacol. 2007;47(9):1104–18.
Spitzer JA. Gender differences in some host defense mechanisms. Lupus. 1999;8(5):380–3.
Terje Andersen J, Bekele Daba M, Berntzen G, Michaelsen TE, Sandlie I. Cross-species binding analyses of mouse and human neonatal fc receptor show dramatic differences in immunoglobulin G and albumin binding. J Biol Chem. 2010;285(7):4826–36.
Trevaskis NL, Kaminskas LM, Porter CJH. From sewer to saviour – targeting the lymphatic system to promote drug exposure and activity. Nat Rev Drug Discov. 2015;14:781–803.
Trincot CE, Caron KM. Lymphatic function and dysfunction in the context of sex differences. ACS Pharmacol Transl Sci. 2019;2(5):311–24.
Varkhede N, Forrest L. Understanding the monoclonal antibody disposition after subcutaneous administration using a minimal physiologically based pharmacokinetic model. J Pharm Pharm Sci. 2018;21(1s):130s–48s.
Wang L, Subasic C, Minchin RF, Kaminskas LM. Drug formulation and nanomedicine approaches to targeting lymphatic cancer metastases. Nanomedicine. 2019;14(12):1605–21.
Yadav P, McLeod VM, Nowell CJ, Selby LI, Johnston APR, Kaminskas LM, et al. Distribution of therapeutic proteins into thoracic lymph after intravenous administration is protein size-dependent and primarily occurs within the liver and mesentery. J Control Release. 2018;272:17–28.
Yang J, Zhang L, Yu C, Yang X-F, Wang H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res. 2014;2:1.
Yip V, Palma E, Tesar DB, Mundo EE, Bumbaca D, Torres EK, et al. Quantitative cumulative biodistribution of antibodies in mice: effect of modulating binding affinity to the neonatal fc receptor. MAbs. 2014;6(3):689–96.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 506 kb)
Rights and permissions
About this article
Cite this article
Kuilamu, E., Subasic, C., Cowin, G.J. et al. Cetuximab Exhibits Sex Differences in Lymphatic Exposure after Intravenous Administration in Rats in the Absence of Differences in Plasma Exposure. Pharm Res 37, 224 (2020). https://doi.org/10.1007/s11095-020-02945-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-02945-2