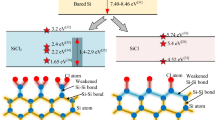
Abstract
Laser desorption of organic compound ions from specially prepared surfaces is known as surface-assisted laser desorption/ionization (SALDI). In this work the properties of a SALDI ion emitter obtained by two-stage laser treatment of crystalline silicon surface have been investigated. The laser surface treatment leads to the formation of a layer with nanoscale objects—quantum dots (QDs) less than 10 nm in size, providing laser desorption of organic compound ions. A change in the desorbing laser wavelength from 351 to 263 nm at comparable laser-exposed spot sizes and fluences results in a sharp decrease in the formation efficiency for MH+ ions and appearance of ions M+ for the same analytes. The effect is apparently determined by the spectral properties of the quantum dots formed on the silicon surface under laser irradiation.





Similar content being viewed by others
REFERENCES
J. Sunner, E. Dratz, and Y.-C. Chen, “Graphite surface-assisted laser desorption/ionization time-of-flight mass spectrometry of peptides and proteins from liquid solutions,” Anal. Chem. 67 (23), 4335–4342 (1995). https://doi.org/10.1021/ac00119a021
P. Kraft, S. Alimpiev, E. Dratz, and J. Sunner, “Infrared, surface-assisted laser desorption ionization mass spectrometry on frozen aqueous solutions of proteins and peptides using suspensions of organic solids,” J. Am. Soc. Mass Spectrom. 9 (9), 912–924 (1998). https://doi.org/10.1016/S1044-0305(98)00063-4
J. A. Stolee, B. N. Walker, V. Zorba, R. E. Russo, and A. Vertes, “Laser–nanostructure interactions for ion production,” Phys. Chem. Chem. Phys. 14 (24), 8453–8471 (2012). https://doi.org/10.1039/C2CP00038E
K. P. Law and J. R. Larkin, “Recent advances in SALDI-MS techniques and their chemical and bioanalytical applications,” Anal. Bioanal. Chem. 399 (8), 2597–2622 (2011). https://doi.org/10.1007/s00216-010-4063-3
A. S. Borodkov, I. I. Kuz’min, N. B. Polyakov, A. A. Grechnikov, and S. S. Alimpiev, “Comparison of the laser desorption/ionization methods for detecting metal complexes,” Phys. Wave Phenom. 25 (4), 243–248 (2017). https://doi.org/10.3103/S1541308X1704001X
J. Li and R. H. Lipson, “Insights into desorption ionization on silicon (DIOS),” J. Phys. Chem. C.117 (51), 27114–27119 (2013). https://doi.org/10.1021/jp4074653
H.-W. Tang, K.-M. Ng, W. Lu, and C.-M. Che, “Ion desorption efficiency and internal energy transfer in carbon-based surface-assisted laser desorption/ionization mass spectrometry: Desorption mechanism(s) and the design of SALDI substrates,” Anal. Chem. 81 (12), 4720–4729 (2009). https://doi.org/10.1021/ac8026367
K. Tanaka, H. Waki, Y. Ido, S. Akita, Y. Yoshida, T. Yoshida, and T. Matsuo, “Protein and polymer analysis up to m/z 100 000 by laser ionization time-of-flight mass spectrometry,” Rapid Commun. Mass Spectrom. 2 (8), 151–153 (1988). https://doi.org/10.1002/rcm.1290020802
K.-M. Ng, S.-L. Chau, H.-W. Tang, X.-G. Wei, K.-C. Lau, F. Ye, and A. M.-C. Ng, “Ion-desorption efficiency and internal-energy transfer in surface-assisted laser desorption/ionization: More implication(s) for the thermal-driven and phase-transition-driven desorption process,” J. Phys. Chem. C.119 (41), 23708–23720 (2015). https://doi.org/10.1021/acs.jpcc.5b05957
X. Wen, S. Dagan, and V. H. Wysocki, “Small-molecule analysis with siliconnanoparticle-assisted laser desorption/ionization mass spectrometry,” Anal. Chem. 79 (2), 434–444 (2007). https://doi.org/10.1021/ac061154l
Z. Shen, J. J. Thomas, C. Averbuj, K. M. Broo, M. Engelhard, J. E. Crowell, M. G. Finn, and G. Siuzdak, “Porous silicon as a versatile platform for laser desorption/ionization mass spectrometry,” Anal. Chem. 73 (3), 612–619 (2001). https://doi.org/10.1021/ac000746f
S. Alimpiev, S. Nikiforov, V. Karavanskii, T. Minton, and J. Sunner, “On the mechanism of laser-induced desorption–ionization of organic compounds from etched silicon and carbon surfaces,” J. Chem. Phys. 115 (4), 1891–1901 (2001). https://doi.org/10.1063/1.1381531
S. N. Zhabin, A. V. Pento, A. A. Grechnikov, A. S. Borodkov, B. G. Sartakov, Ya. O. Simanovsky, S. M. Nikiforov, and S. S. Alimpiev, “On the role of laser irradiation in the processes of laser desorption/ionisation from silicon surfaces,” Quantum Electron. 41 (9), 835–842 (2011). https://doi.org/10.1070/Qe2011v041n09abeh014678
S. Alimpiev, A. Grechnikov, J. Sunner, V. Karavanskii, Ya. Simanovsky, S. Zhabin, and S. Nikiforov, “On the role of defects and surface chemistry for surface-assisted laser desorption ionization from silicon,” J. Chem. Phys. 128 (1), 014711 (2008). https://doi.org/10.1063/1.2802304
E. P. Go, J. V. Apon, G. Luo, A. Saghatelian, R. H. Daniels, V. Sahi, R. Dubrow, B. F. Cravatt, A. Vertes, and G. Siuzdak, “Desorption/ionization on silicon nanowires,” Anal. Chem. 77 (6), 1641–1646 (2005). https://doi.org/10.1021/ac048460o
J. A. Stolee and A. Vertes, “Polarization dependent fragmentation of ions produced by laser desorption from nanopost arrays,” Phys. Chem. Chem. Phys. 13 (20), 9140–9146 (2011). https://doi.org/10.1039/C0CP02709J
S. Alimpiev, A. Grechnikov, J. Sunner, A. Borodkov, V. Karavanskii, Ya. Simanovsky, and S. Nikiforov, “Gas chromatography/surface-assisted laser desorption ionization mass spectrometry of amphetamine-like compounds,” Anal. Chem. 81 (3), 1255–1561 (2009). https://doi.org/10.1021/ac802176j
A. A. Grechnikov, A. S. Borodkov, S. S. Alimpiev, S. M. Nikiforov, and Ya. O. Simanovsky, “Gas-phase basicity: Parameter determining the efficiency of laser desorption/ionization from silicon surfaces,” J. Anal. Chem. 68 (1), 19–26 (2013). https://doi.org/10.1134/S1061934812110056
Y. Chen and A. Vertes, “Adjustable fragmentation in laser desorption/ionization from laser-induced silicon microcolumn arrays,” Anal. Chem. 78 (16), 5835–5844 (2006). https://doi.org/10.1021/ac060405n
S. S. Alimpiev, S. M. Nikiforov, A. A. Grechnikov, and J. A. Sunner, “Novel technique for ultra sensitive detection of organic compounds,” in Vapour and Trace Detection of Explosives for Anti-Terrorism Purposes, NATO Science Series (Ser. II: Mathematics, Physics and Chemistry), Ed. by M. Krausa and A. A. Reznev (Springer, Dordrecht, 2004), Vol. 167, p. 101–112. https://doi.org/10.1007/978-1-4020-2716-1_12
A. A. Grechnikov, S. S. Alimpiev, S. M. Nikiforov, and Ya. O. Simanovsky, RF Patent No. 2426191, Byull. Izobret., No. 22 (2011).
W. C. Wiley and I. H. McLaren, “Time-of-flight mass spectrometer with improved resolution,” Rev. Sci. Instrum. 26 (12), 1150–1157 (1955). https://doi.org/10.1063/1.1715212
S. G. Lias, “Gas phase ion energetics data,” in NIST Chemistry WebBook—NIST Standard Reference Database Number 69, Eds. P. J. Linstrom and W. G. Mallard (Nat. Inst. Standards and Technol., Gaithersburg MD, 2020) (retrieved February 13, 2020). https://doi.org/10.18434/T4D303
E. P. L. Hunter and S. G. Lias, “Evaluated gas phase basicities and proton affinities of molecules: An update,” J. Phys. Chem. Ref. Data. 27 (3), 413–656 (1998). https://doi.org/10.1063/1.556018
V. Talrose, E. B. Stern, A. A. Goncharova, N. A. Messineva, N. V. Trusova, and M. V. Efimkina, “UV/visible spectra,” in NIST Chemistry WebBook—NIST Standard Reference Database Number 69, Eds. P. J. Linstrom and W. G. Mallard (Nat. Inst. Standards and Technol., Gaithersburg MD, 2020) (retrieved February 13, 2020). https://doi.org/10.18434/T4D303
D. Dougherty, E. S. Younathan, R. Voll, S. Abdulnur, and S. P. McGlynn, “Photoelectron spectroscopy of some biological molecules,” J. Electron Spectrosc. Relat. Phenom. 13 (3), 379–393 (1978). https://doi.org/10.1016/0368-2048(78)85042-7
H. Bahrami, M. Tabrizchi, and H. Farrokhpour, “Protonation of caffeine: A theoretical and experimental study,” Chem. Phys. 415, 222–227 (2013). https://doi.org/10.1016/j.chemphys.2013.01.022
A. Belay, K. Ture, M. Redi, and A. Asfaw, “Measurement of caffeine in coffee beans with UV/vis spectrometer,” Food Chem. 108 (1), 310–315 (2008). https://doi.org/10.1016/j.foodchem.2007.10.024
M. A. Smith, J. W. Hager, and S. C. Wallace, “Two color photoionization spectroscopy of jet cooled aniline: Vibrational frequencies of the aniline \({{\tilde {X}}^{2}}{{B}_{1}}\) radical cation,” J. Chem. Phys. 80 (7), 3097–3105 (1984). https://doi.org/10.1063/1.447124
J. E. Mathis and R. N. Compton, “Single and multiple photon ionization of triethylamine,” J. Chem. Phys. 104 (21), 8341–8347 (1996). https://doi.org/10.1063/1.471585
M. Berton, R. Mello, R. Acerete, and M. E. González Núñez, “Photolysis of tertiary amines in the presence of CO2: The paths to formic acid, α-amino acids, and 1,2-diamines,” J. Org. Chem. 83 (1), 96–103 (2018). https://doi.org/10.1021/acs.joc.7b02407
H. R. Wendt and H. E. Hunziker, “The UV spectra of primary, secondary, and tertiary alkyl radicals,” J. Chem. Phys. 81 (2), 717–723 (1984). https://doi.org/10.1063/1.447755
D. E. Aspnes and A. A. Studna, “Dielectric functions and optical parameters of Si, Ge, GaP, GaAs, GaSb, InP, InAs, and InSb from 1.5 to 6.0 eV,” Phys. Rev. B.27 (2), 985–1009 (1983). https://doi.org/10.1103/PhysRevB.27.985
J.-S. Chen, M. Li, and M. Cotlet, “Nanoscale photoinduced charge transfer with individual quantum dots: Tunability through synthesis, interface design, and interaction with charge traps,” ACS Omega. 4 (5), 9102–9112 (2019). https://doi.org/10.1021/acsomega.9b00803
N. V. Tkachenko, “Photoinduced charge separation in semiconductor-quantum-dot/organic-molecule hybrids,” ChemPhotoChem. 2 (3), 112–120 (2018). https://doi.org/10.1002/cptc.201700161
P. L. Liu, R. Yen, N. Bloembergen, and R. T. Hodgson, “Picosecond laser-induced melting and resolidification morphology on Si,” Appl. Phys. Lett.34 (12), 864–865 (1979). https://doi.org/10.1063/1.90703
S. Watanabe, Y. Yoshida, S. Kayashima, S. Yatsu, M. Kawai, and T. Kato, “In situ observation of self-organizing nanodot formation under nanosecond-pulsed laser irradiation on Si surface,” J. Appl. Phys. 108 (10), 103510 (2010). https://doi.org/10.1063/1.3512888
M. Han and J. Sunner, “An activated carbon substrate surface for laser desorption mass spectrometry,” J. Am. Soc. Mass Spectrom. 11 (7), 644–649 (2000). https://doi.org/10.1016/S1044-0305(00)00129-X
C. Bulutay, “Interband, intraband, and excited-state direct photon absorption of silicon and germanium nanocrystals embedded in a wide band-gap lattice,” Phys. Rev. B.76 (20), 205321 (2007). https://doi.org/10.1103/PhysRevB.76.205321
M. Dasog, Z. Yang, S. Regli, T. M. Atkins, A. Faramus, M. P. Singh, E. Muthuswamy, S. M. Kauzlarich, R. D. Tilley, and J. G. C. Veinot, “Chemical insight into the origin of red and blue photoluminescence arising from freestanding silicon nanocrystals,” ACS Nano. 7 (3), 2676–2685 (2013). https://doi.org/10.1021/nn4000644
Q. Li and R. Jin, “Photoluminescence from colloidal silicon nanoparticles: Significant effect of surface,” Nanotechnol. Rev. 6 (6), 601–612 (2017). https://doi.org/10.1515/ntrev-2017-0145
L. Patrone, D. Nelson, V. I. Safarov, M. Sentis, W. Marine, and S. Giorgio, “Photoluminescence of silicon nanoclusters with reduced size dispersion produced by laser ablation,” J. Appl. Phys. 87 (8), 3829–3837 (2000). https://doi.org/10.1063/1.372421
D. Menzel and R. Gomer, “Desorption from metal surfaces by low-energy electrons,” J. Chem. Phys. 41 (11), 3311–3328 (1964). https://doi.org/10.1063/1.1725730
D. Menzel, “Thirty years of MGR: How it came about, and what came of it,” Nucl. Instrum. Methods Phys. Res.,Sect. B.101 (1-2), 1–10 (1995). https://doi.org/10.1016/0168-583X(95)00060-7
Funding
This study was supported in part by the Russian Foundation for Basic Research, project no. 18-32-01018.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by Yu. Sin’kov
About this article
Cite this article
Pento, A.V., Nikiforov, S.M. & Simanovsky, Y.O. Laser Desorption of Organic Compound Ions from a Silicon Surface Modified by Laser Irradiation. Phys. Wave Phen. 28, 213–221 (2020). https://doi.org/10.3103/S1541308X20030164
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1541308X20030164