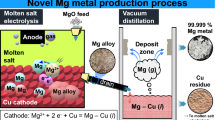
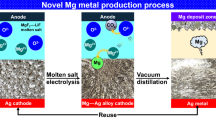
Abstract
A novel electrolytic process that uses a liquid–metal cathode was investigated to produce high-purity magnesium (Mg) metal using magnesium oxide (MgO). Electrolysis of MgO in a magnesium fluoride (MgF2)-lithium fluoride (LiF) molten salt was carried out with applied voltages of 2.5 V to 3.0 V at 1053 K to 1083 K using tin (Sn), silver (Ag), or copper (Cu) as the cathode and graphite or platinum (Pt) as the anode. After electrolysis, Mg alloys with Mg2Sn, AgMg, and Cu2Mg phases were produced with current efficiencies of 77.2 to 83.8 pct when the concentration of Mg in the Mg alloys was 11.9 to 12.9 mass pct. For the production of high-purity Mg metal directly from the Mg alloys, vacuum distillation was performed. When vacuum distillation was conducted at 1200 K to 1300 K, the concentration of Mg in the Mg alloys feed decreased from 30.2 to 34.1 mass pct to 0.32 to 1.75 mass pct, and Mg metal with a purity of 99.975 to 99.999 pct was obtained. Therefore, this study demonstrates that the production of high-purity Mg metal through an efficient and environmentally sound method using the electrolysis of MgO is feasible.














Similar content being viewed by others
References
C. Zhong, F. Liu, Y. Wu, J. Le, L. Liu, M. He, J. Zhu, and W. Hu: J. Alloy. Compd, 2012, vol. 520, pp. 11–21.
Roskill: Magnesium Metal: Global Industry, Markets and Outlook to 2020, 12th ed., Roskill Information Services Ltd., London, 2016.
U.S. Department of Energy: Materials Technologies: Goals, Strategies, and Top Accomplishments, 2010, https://www.enegy.gov/sites/prod/files/2014/03/f13/materials_tech_goals.pdf.
M. Tauber: Proceedings of 76th International Magnesium Association World Magnesium Conference, Int. Magnesium Ass., Budapest, Hungary, 2019, pp. 1–16.
Y. Wada, S. Fujii, E. Suzuki, M. M. Maitani, S. Tsubaki, S. Chonan, M. Fukui, and N. Inazu: Sci. Rep., 2017, vol. 7, pp. 1-7.
L.M. Pidgeon and W.A. Alexander: T. Am. I. Min. Met. Eng., 1944, vol. 159, pp. 315-52.
H.E. Friedrich and B.L. Mordike: Magnesium Technology: Metallurgy, Design Data, Applications, Springer-Verlag Berin Heidelberg, Berin, 2006.
G. Holcroft: Proceedings of 75th International Magnesium Association World Magnesium Conference, Int. Magnesium Ass., New Orleans, USA, 2018, pp. 239-47.
R.L. Thayer and R. Neelameggham: JOM, 2001, vol. 53, pp. 15-17.
G.T. Mejdell, H.M. Baumann, and K.W. Tveten: Method for Production of Magnesium Chloride, US 5112584, 1992.
G.J. Kipouros and D.R. Sadoway: Advances in Molten Salt Chemistry 6, Elsevier Science, Amsterdam, 1987, pp. 127-209.
D. Chunming: Proceedings of 76th International Magnesium Association World Magnesium Conference, Int. Magnesium Ass., Budapest, Hungary, 2019, pp. 86–113.
R.A. Sharma: JOM, 1996, vol. 43, pp. 39-43.
N. Leonard, M. Korenko, C. Larson, K. Blood, L.J. Venstrom, S. Nudehi, S. Duncan, R. Diver, F. Simko, J. Priscak, J. Schoer, P.T. Kissinger, and R. Palumbo: Chem. Eng. Sci., 2016, vol. 148, pp. 155-69.
A. Krishnan, X.G. Lu, and U.B. Pal: Metall. Mater. Trans. B, 2005, vol. 36, pp. 463-73.
A.C. Powell IV and S.J. Derezinski III: Primary Production of Elements, US 8460535 B2, 2013.
X. Guan, U.B. Pal, and A.C. Powell: Metall. Mater. Trans. E, 2014, vol. 1A, pp. 132-44.
A.C. Powell IV: Final Scientific/Technical Report of Industrial Scale-Up of Low-Cost Zero-Emission Magnesium by INFINIUM Electrolysis, 2018. https://www.osti.gov/biblio/1431302.
L.A. Yerkes: Electrolytic Method for Producing Magnesium Alloys, US 2431723, 1942.
I. Barin: Thermochemical Data of Pure Substances, 3rd ed., VCH Verlagsgesellschaft mbH, Weinheim, 1995.
C.W. Bale, E. Belisle, P. Chartrand, S.A. Decterov, G. Eriksson, A.E. Gheribi, K. Hack, I.H. Jung, Y.B. Kang, J. Melancon, A.D. Pelton, S. Petersen, C. Robelin, J. Sangster, P. Spencer, and M-A. V. Ende: Calphad, 2016, vol. 54, pp. 35–53.
J. Kang and T.H. Okabe: Metall. Mater. Trans. B., 2014, vol. 45, pp. 1260-71.
L. Kong, T. Ouchi, C. Zheng, and T.H. Okabe: J. Electrochem. Soc., 2019, vol. 166, pp. E429-37.
T.B. Massalski: Bianry Alloy Phase Diagrams, 2nd ed., ASM International, Materials Park, Geauga County, 1990.
The Japan Magnesium Association: Handbook of Advanced Magnesium Technology, Kallos Publishing Co., LTD., Tokyo, 2000.
O. Takeda, T. Uda, and T.H. Okabe: Treatise on Porcess Metallurgy, vol. 3, Elsevier, Amsterdam, 2014, pp. 995-1069.
Acknowledgments
The authors are grateful to Dr. DongEung Kim of the Korea Institute of Industrial Technology for the discussions throughout this study. In addition, the authors also thank Ms. Gyeonghye Moon, Ms. Jiyoung Baek, Dr. Jae-Yeol Yang, and Dr. Jae-Sik Yoon for their technical support. Furthermore, the authors are grateful to all the members of the Geoanalysis Department of KIGAM for their technical assistance. This research was supported by the National Research Council of Science & Technology (NST) Grant by the Korea government (MSIT) (No.CRC-15-06-KIGAM).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted May 10, 2020, Accepted September 11, 2020.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, TH., Okabe, T.H., Lee, JY. et al. Molten Salt Electrolysis of Magnesium Oxide Using a Liquid–Metal Cathode for the Production of Magnesium Metal. Metall Mater Trans B 51, 2993–3006 (2020). https://doi.org/10.1007/s11663-020-01976-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-020-01976-9