1. Introduction
Bioreactors are defined as facilities that enable the efficient operation of microbiological processes by controlling culture parameters and managing its optimal conditions while limiting the possibilities of its contamination [
1,
2] Bioreactors are the main part of any biotechnological processes in which living microorganisms are applied to produce a wide range of desired bioproducts with maximal efficiency. Optimization of the biotechnological processes is directly linked to intensive research work in the field of construction equipment. The basic features of various types of bioreactors are described in
Table 1.
In general, the industrial production may be carried out using bioreactors divided into three groups: (i) non-stirred and non-aerated systems (~76% of all bioreactors); (ii) non-stirred but aerated systems (~11% of all bioreactors); (iii) stirred and aerated systems (~13% of all bioreactors) [
3] The last group of bioreactors (aerated and stirred vessels) can be called standard bioreactors. These stirred-tank bioreactors are equipped with a different type of impeller or impellers for mixing culture media and a sparger for delivering oxygen to the cells. The conventional mixing vessel has two main advantages: a low investment capital required to set up the bioreactor and low operating costs. These systems allow full automation and the execution of bioprocess in closed systems that can be monitored and maintained at defined operational conditions.
The nutrients concentration and bioreactor operation modes influence cell growth rates and the number of products synthesized within the bioprocess. They can influence cell growth and metabolite formation in cell factories, leading to substantially differentiated productivities. In general, the bioreactors can be classified as a tank, tubular, and column devices that operate in various modes, including batch, semi-continuous, fed-batch, and continuous operation. The operating parameters in a bioreactor depend on the type of operation. The main operating parameters characteristic to the three above-mentioned modes of bioreactor operation are presented in
Table 2.
Many reactor systems for the bioproduction’s have been developed in recent years. Many of these systems are commercially available. The BioFlo
® bioreactors are easy to use, entry-level systems equipped with built-in controls that can operate as a microbiological or cell culture reactor. This paper focuses on the performance of two BioFlo
® systems (BioFlo
® 115 and BioFlo
® 415), considering both mixing properties and mass transfer ratio of these multi-impeller systems.
Table 3 compares BioFlo
® 115 and BioFlo
® 415 bioreactors.
The BioFlo® systems may be treated as fully baffled bioreactors stirred by two six-blade Rushton turbines. The unequal distribution of shear forces and energy dissipation of mixed fluid is the main flaw that characterizes the standard single-impeller stirred tanks. Nevertheless, these types of forces generated by a single impeller may be useful when the destruction of the mixed multiphase structure is required in production processes. Such conditions may affect microorganisms and, therefore, the entire bioprocess. On the other hand, the shear-forces-mediated stress produced by multi-impeller systems is particularly important for biochemical processes. Moreover, the multi-impeller mixing system of the BioFlo® leads to more efficient gas distribution and gas phase residence time by an increased hold-up. Additionally, this system is characterised by a lower power consumption per impeller in comparison to single-impeller systems. These systems may also be used to avoid the dead zones formation in the mixed fluid.
The studies on hydrodynamics have been focused in recent decades on a better understanding of the complex effects generated by two Rushton-impeller-stirred tanks. Practical applications of the double-Rushton mixer have been researched for several years [
4,
5,
6,
7,
8,
9,
10] The comparison of the multiple- and the single-impeller-based agitation systems with a special focus on their bioprocessing applications was presented in Ref. [
11].
The mixing intensities in the case of dual-impeller-based bioreactors of the dual-impeller system comprising either two Rushton impellers or one Rushton and one axial flow impeller were presented in Ref. [
12] Furthermore, the application of the Rushton impeller combined with axial flow impellers in bioreactors was discussed in Ref. [
13] in the yeast cultivation model. The experimental studies concerning the mixing of non-aerated bacterial, yeasts, and fungal cultures in a bioreactor stirred with a double turbine impeller were presented in Ref. [
14]. The pH-response-based method of the mixing time measurement was applied by Hadjiev et al. [
15] where the influence of various parameters on the dimensionless mixing time in an aerated, dual-Rushton-stirred, laboratory-scale reactor was investigated. Moreover, Bouaifi and Roustan [
16] discussed the experimental results on the power consumption, mixing time, and mixing energy in a non-standard vessel equipped with various axial and mixed dual-impeller configurations. The mixing characteristics (e.g., mixing time, homogenization energy, and residence time distribution) of a gas-induced reactor with two 45°-pitched downward blade turbines were analyzed by Jafari and Mohammadzadeh [
17]. In addition, oxygen mass transfer characteristics for various twin- and single-impeller systems (e.g., Rushton turbine, pitched 4-, and 2-blade impellers) were studied by Karimi et al. [
18], while the power consumption and the mixing time for the non-baffled agitated vessel with a double impeller were measured by Hiraoka et al. [
19], who later discussed the best set-up position and the combination of impellers. Many effects, including floating solids concentrations, the diameter of the impeller used, and the off-bottom clearance of the impeller were analyzed in Ref. [
20] They also discussed the spacing between impellers concerning the critical speed of the impeller, power consumption, and mixing time. These were all recorded in the dual-impeller agitated vessel. Moreover, Woziwodzki et al. [
21] indicated that the viscosity can also have an effect of the mixing parameters, which they measured in a system containing a dual eccentrically located impeller.
Based on the literature search in scientific databases (Scopus, Sciencedirect, NCBI), we assume that the research concerning the analysis of the mixing process in BioFlo® systems has not yet been performed. Therefore, we aimed at analyzing the power consumption, mixing time and energy (or homogenization energy), and mass transfer coefficient for the BioFlo® 115 and BioFlo® 415 bioreactors used under gassed and ungassed conditions.
2. Experimental Set-Up
A widespread laboratory-scale stirred dual-Rushton bioreactor system marketed by BioFlo
® was used in the experiments. Schematic design and the main geometrical parameters of BioFlo
® 115 (Eppendorf, Enfield, CT, USA) and BioFlo
® 415 (Eppendorf, Enfield, CT, USA) apparatus are presented in
Figure 1 and
Figure 2, respectively.
The measurements of the mixing process for BioFlo® 115 were performed in a cylindrical glass vessel with the liquid height-to-vessel-diameter ratio equal to 1.45 (HL = 180 mm; D = 124.5 mm; working volume V = 2 dm3). In the case of BioFlo® 415, the ratio was equal to 2.31 (HL = 345 mm; D = 149.5 mm; working volume V = 5.5 dm3).
Distilled water served as the experimental liquid. The mixing process in both BioFlo® systems was carried out under different values of dual-Rushton turbine rotation speed (n = 0.33–10 s−1). The dimensionless Reynolds number, , calculated for BioFlo® 115 and BioFlo® 415 was varying in the range 900–27,000 and 2930–35,150, respectively.
3. Flow Patterns in BioFlo® Systems
The visual representation of fluid flow patterns in a stirred vessel can be generated by the use of the computational fluid dynamics (CFD) technique. The numerical simulations, with the help of CFD codes, give access to qualitative and quantitative parameters describing the mixing performance and hydrodynamics of the agitated systems. The numerical methods have led to the development of differential equations and have built distributed parameter models that are also spatially and temporally representative for the tested systems. The possibility of using CFD for the numerical simulation of the flow distribution in a mixer can significantly contribute to the understanding of the mixing processes and to providing better, faster, and cheaper optimization design [
22]. This study utilized the CFD method to describe the mixing process and to analyze velocity profiles and flow patterns in the BioFlo
® systems.
The geometry of the bioreactors was generated using AutoCAD software. (An identical computational model of geometries was created, which consists of 1.8 and 1.9 million tetrahedral volume elements for BioFlo
® 115 and BioFlo
® 415, respectively.) These geometries were imported to Design Modeler and introduced to ANSYS Meshing software to generate a CFD mesh. In the next step, the commercial CFD package ANSYS Workbench 14.5 was used to calculate the liquid flow based on the numerical computations. Flow patterns were calculated using ANSYS CFX software. The control-volume formulation was used to solve the equations of mass and momentum conservation. Reynolds-averaged Navier-Stokes (RANS) equations with the
k-ω turbulence-closure model could be successfully solved with the three-dimensional finite volume CFD code. Applying appropriate corrections in the numerical approach allowed us to compute laminar and turbulent flows in the static mixers. The basic two-equations
k-ω model was first introduced by Wilcox [
23]. In our variant, we have introduced modifications in the model for low-Reynolds number effects, as well as compressibility and shear flow spreading. The multiple reference frame (MRF), which is a common approach in turbomachinery modelling (e.g., mixers), was employed during the CFD analysis. It consists of at least two zones—stationary and rotating (around the impeller). Specific equations were calculated inside each zone and data were exchanged at the interface. Such an approach assumed no relative motion; thus, it was similar to analyzing the instantaneous flow field with the impeller in its freeze position. The rest of the boundary conditions were set to walls, and only the liquid top surface was set with the degassing conditions.
The typical computational velocity profiles for the tested bioreactors are presented in
Figure 3. This figure shows the differences between the flow field calculated at
N = 50, 200, 400 and 600 rpm, respectively. To ease the analysis of the three-dimensional hydrodynamics, the velocity was shown as the two-dimensional scheme. When the residuals for equations of continuity and momentum reached a value below 10
−7, the convergence was assumed in each simulation.
It is well known that impeller spacing may affect the flow pattern in the stirred tanks [
24]. For the double-impeller agitator, the flow pattern strongly depends on the geometry combinations (e.g., clearance from the bottom and above the upper impeller, as well as the reactor and impeller geometry) and the mutual influence of the two impellers. Moreover, the observed flow patterns were influenced by impeller characteristics, the presence of the baffles, and the additional tubes.
The use of two Rushton turbines developed independent flow patterns characterized by greater impeller clearance than the impeller diameter [
9]. In the case of this study, the impeller spacing and the impeller diameter ratios were equal to 1.46 and 3.15 for BioFlo
® 115 and BioFlo
® 415, respectively. Therefore, each turbine was characterized by a parallel flow pattern where specific upper and lower ring vortices led to four stable ring vortices formation. It should be noted that impellers produce a radial jet outward divided into the near-wall streams. Additionally, the high shear forces and the rapidly mixing turbulent regions were generated near the impellers. These regions could be responsible for the enhancement in mass transfer in the gas–liquid systems. Moreover, the correct assignment of the strong mixing regions inside the bioreactor may be essential for the development of bioprocesses that use cells sensitive to shear forces.
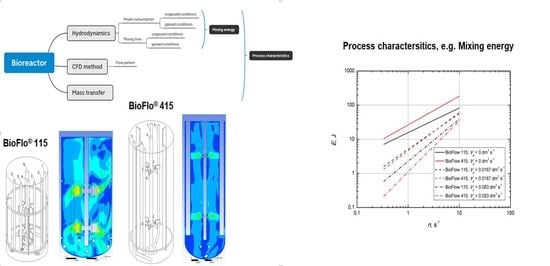
6. Mixing Energy
The power consumption and mixing time can be determined form the adequate analytical correlation (ungassed conditions: power consumption—Equations (2) and (3)—and mixing time—Equation (13) (
Figure 7); gassed conditions: power consumption—Equations (9) and (10)—and mixing time—Equations (16) and (17)).
The general formula for this parameter was assumed as follows:
This magnitude can be used to calculate the production costs, which have a significance in the economic analysis. The effect of the impeller speed on the mixing efficiency is shown in
Figure 10.
The mixing energy plot shows an increasing trend with the impeller speed for the tested BioFlo® mixing systems under ungassed and gas conditions. The comparison indicates that the dimensional mixing energy under ungassed conditions was on average ~95% higher in the case of BioFlo® 415.
The data depicted in
Figure 10 indicate that BioFlo
® 415 was more effective for mixing liquid under the gassed conditions. This advantage could be observed in the whole mixing intensity region. The discrepancy of the dimensional mixing energy increased with the increasing gas flow rate. The mixing energy under gassed conditions for BioFlo
® 115 was consequently higher than the data obtained for the second reactor. For example, the data for BioFlo
® 115 with the gas flow rate
equal to 0.0167 and 0.083 dm
3∙s
−1 are on average ~10% and ~60% higher than the mixing energy for BioFlo
® 415.
The mixing efficiency of different types of mixing systems may be compared using the values of mixing energy. Bouaifi and Roustan [
16] investigated the power consumption, mixing time, and mixing efficiency in a vessel equipped with various axial and mixed dual-impeller configurations. The results obtained from these preliminary studies are comparable with the data obtained for the reactors.
Figure 11 compares the experimental data of the mixing energy for the used mixing systems. This comparison is presented for a rotational speed
n = 8.33 s
−1 (these data were presented in the paper published by Bouaifi and Roustan). As shown in
Figure 11, the usage of BioFlow
® 115 and BioFlow
® 415 correspond to the lowest mixing energy. This figure shows a clear trend of decreasing mixing energy for the tested mixing system with increasing gas flow. It should be noted that the highest mixing efficiency corresponds to the minimum energy consumption.
7. Mass Transfer Characteristics
The volumetric gas–liquid mass transfer coefficient (kLa) belongs to the group of global parameters dependent on the impeller design, its configuration, geometrical parameters of the mixer, power draw, and properties of the gas–liquid system. This magnitude defines the efficiency of mass transfer ratio (in this case, gas transfer) within the bioreactor. From the practical point of view, the oxygen transfer rate in nitrogen-purged liquid (distilled water) or dissolved oxygen probe is used to obtain the gas–liquid transfer coefficient.
In the case of these investigations, the oxygen electrode was used to measure the concentrations of dissolved oxygen. The changes in this parameter were recorded with the central unit of BioFlo
® systems. The
kLa coefficient was determined from the plotted graph
against time,
t. This coefficient is the slope of the graph as follows:
The calculated data of the volumetric gas–liquid mass transfer coefficient for the tested mixing systems are given by the relationship of the form given by:
The influence of the operational parameters on the mass transfer coefficient may be written in the following form (the constants and the exponents in the obtained relationships were computed employing the Matlab software):
Figure 12 depicts the variation of the mass transfer coefficient with the specific power draw (power consumption under gassed conditions per unit volume) at the various gas flow rate values.
The mass transfer coefficient increases with an increase in the power input per volume unit. The obtained results showed that the mass transfer coefficient was strongly dependent on the gas flow rate. The mass transfer coefficient’s lowest values were ascribed to BioFlo® 415.
8. Summary
This paper’s objective was to study the mixing performance of BioFlo® 115 and BioFlo® 415 using experimental and computational methods. From the chemical engineering perspective, these bioreactors may be treated as vessels equipped with a dual-impeller system. It should be noted that BioFlo® 115 and BioFlo® 415 are stirred-tank gas–liquid reactors used for carrying out the cultivation of cells and enzymatic processes, mainly in batch operations. In stirred-tank bioreactors, mixing by agitation and/or bubbling of a gas stream is influenced by the hydrodynamic conditions in the mixed liquid, which directly affects the bioprocessing. The present study was designed to determine the hydrodynamic parameters (flow field, specific power input, mixing energy as a product of power consumption and mixing time) and mass transfer process (gas–liquid transfer). The current study’s main goal was to determine the mathematical relationships, which may be applied for the analysis of the mixing process in the tested bioreactors. This research indicates that gassed conditions have a strong influence on the analyzed hydrodynamic parameters.
These findings suggest that the applied dual-impeller mixing systems influence power consumption under ungassed conditions. Introducing the gas stream into the bioreactors vessels results in a decrease in power consumption. It was also shown that this effect is stronger in BioFlo® 115.
The second major finding was that mixing energy as the product of power consumption and mixing time could serve as an important criterion in the analysis of the mixing process in bioreactors. For the first time, this study has demonstrated that the mixing energy may be applied to determine the mixing efficiency of commercial bioreactors. This experimental work shows that the application of gassed conditions increases the mixing efficiency. The improvement of the mixing process enables a decrease in the mixing energy. The present study confirms that BioFlow® 115 and BioFlow® 415 may be successfully applied in bioprocesses. It has been found that the tested dual-impeller mixing systems consume much less energy in comparison with the dual-impellers systems (e.g., A-315+A-31; A-315+A-310; A-315+PBTD; RTD+A-315; RTD+A-310; RTD+PBTD). The obtained results suggest that the tested bioreactors offer profitable mass transfer performance. The volumetric gas-liquid mass transfer coefficient for BioFlo® 115 is higher than that observed in the case of BioFlo® 415 bioreactors.