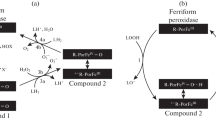
Abstract
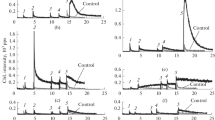
This study deals with participation of isoquinoline derivatives of coumarin in the peroxidase reaction catalyzed by the cytochrome c–cardiolipin complex. We have studied coumarin derivatives called coumarin-314 (C-314), coumarin-334 (C-334) and coumarin-525 (C-525). These substances are considered as specific physical activators of chemiluminescence, which accompanies the lipid peroxidation reaction. In the course of the scientific research, a spectrophotometric study of the effect of methanol on the structure of cytochrome c was performed, the optical properties of these substances were studied in the medium of a phosphate buffer, and a spectrophotometric study was performed with parallel registration of the chemiluminescence of a mixture in which cytochrome c catalyzed by a complex with cardiolipin lipoperoxidase reaction in the presence of isoquinolysin derivatives of coumarin. The conclusions of this work contain identification of the reversibility of the action of methanol on the structure of cytochrome c, which proves the possibility of using this alcohol in the study of this protein, the positions of absorption maxima and corresponding values of molar absorption coefficients in the medium of 20 mM phosphate buffer (pH = 7.4) for C-314 (λmax = 447.5 nm; ε = 32360.4 L/(mol cm)), C-334 (λmax = 460 nm; ε = 44 012 L/(mol cm)), and C-525 (λmax = 460 nm; ε = 32 703.56 L/(mol cm)). We also demonstrated that the said substances are substrates of cytochrome c/cardiolipin complex-catalyzed peroxidase reaction. Mass flow of these substances during an average statistical experiment in measurement of chemiluminescence (322 s) for C-314, C-334 and C-525 amounted to 31.89, 37.61 and 25.94%, respectively.







Similar content being viewed by others
REFERENCES
T. Nishikido, J. Oyama, A. Shiraki, et al., J. Am. Heart Assoc. 5 (4), e002863 (2016). https://doi.org/10.1161/JAHA.115.002863
G. L. Caldeira, I. L. Ferreira, and A. C. Rego, J. Alzheimers Dis. 34, 115 (2013). https://doi.org/10.3233/JAD-121444
H. H. Gaballah, S. S. Zakaria, M. M. Elbatsh, and N. M. Tahoon, Chem. Biol. Interact. 251, 10 (2016). https://doi.org/10.1016/j.cbi.2016.03.023
L. Fan, L. Jiang, and Z. Du, Metab. Brain Dis. 30, 1269 (2015). https://doi.org/10.1007/s11011-015-9703-z
N. Yalcinkaya, H. Haytural, B. Bilgic, et al., Neurosci. Lett. 615, 72 (2016). https://doi.org/10.1016/j.neulet.2016.01.029
E. V. Proskurnina, Yu. A. Vladimirov, and A. M. Polimova, in Neuroscience for Medicine and Psychology: Proc. 11 Int. Interdisc. Conf. (Sudak, 2015), p. 309 [in Russian].
Yu. A. Vladimirov, E. V. Proskurnina, D. Yu. Izmailov, et al., Biochemistry (Moscow) 74 (3), 301 (2009).
Yu. A. Vladimirov, E. V. Proskurnina, D. Yu. Izmailov, et al., Biochemistry (Moscow) 71 (9), 998 (2006).
Yu. A. Vladimirov, E. V. Proskurnina, D. Yu. Izmailov, et al., Biochemistry (Moscow) 91 (8), 1019 (2006).
A. N. Osipov, G. O. Stepanov, Yu. A. Vladimirov, et al., Biochemistry (Moscow) 71 (10), 1218 (2006).
E. M. Demin, E. V. Proskurnina, and Yu. A. Vladimirov, Moscow Univ. Chem. Bull. 63 (5), 297 (2008).
Yu. A. Vladimirov, E. M. Demin, E. V. Proskurnina, A. N. Osipov, Biochemistry (Moscow), Ser. A: Membr. Cell Biol. 3 (4), 467 (2009).
E. M. Demin, D. Yu. Izmailov, E. V. Proskurnina, and Yu. A. Vladimirov, Regulation of the Radical-dependent Stage of Apoptosis by Antioxidants (MAKS-Press, Moscow, 2012) [in Russian].
Yu. A. Vladimirov, in Medical and Biological Applications of Lasers: Proc. 40th Int. Sci.-Pract. Conf. (Yalta, 2013), p. 174 [in Russian].
Yu. A. Vladimirov, E. V. Proskurnina, and A. V. Alekseev, Biochemistry (Moscow) 78 (10), 1086 (2013).
E. M. Demin, E. V. Proskurnina, Yu. A. Vladimirov, in Proc. 2nd Russian Congress of Analysts (Moscow, 2013), p. 275 [in Russian].
A. S. Vikulina, A. V. Alekseev, E. V. Proskurnina, and Yu. A. Vladimirov, Biochemistry (Moscow) 80 (10), 1298 (2015).
V. E. Kagan, V. A. Tyurin, J. Jiang, et al., Nature Chem. Biol. 1, 223 (2005).
N. A. Belikova, Y. A. Vladimirov, A. N. Osipov, et al., Biochemistry 45, 4998 (2006).
L. Milazzo, L. Tognaccini, B. D. Howes, et al., Biochemistry 56, 1887 (2017). https://doi.org/10.1021/acs.biochem.6b01281
Y. A. Vladimirov, C. Sarisozen, G. K. Vladimirov, et al., Pharm. Res. 34, 1264 (2017). https://doi.org/10.1007/s11095-017-2143-1
D. A. Capdevila, S. Oviedo Rouco, F. Tomasina, et al., Biochemistry 54, 7491 (2015). https://doi.org/10.1021/acs.biochem.5b00922
H. Kobayashi, S. Nagao, and S. Hirota, Angew Chem. Int. Ed. Engl. 55, 14019 (2016). https://doi.org/10.1002/anie.201607419
A. Bhujade, G. Gupta, S. Talmale, et al., Food Funct. 4, 338 (2013). https://doi.org/10.1039/c2fo30167a
D. A. Vasina, D. D. Zhdanov, E. V. Orlova, et al., Biochemistry (Moscow) 82, 24 (2017). https://doi.org/10.1134/S0006297917010035
S. Li, T. Wang, L. Zhai, et al., J. Mol. Neurosci. 64, 144 (2018). https://doi.org/10.1007/s12031-017-1012-z
P. Bemani, M. Mohammadi, and A. Hakakian, Asian Pac. J. Cancer Prev. 19, 97 (2018). https://doi.org/10.22034/APJCP.2018.19.1.97
S. A. Susin, E. Daugas, L. Ravagnan, et al., J. Exp. Med. 192, 571 (2000).
Yu. A. Vladimirov and A. I. Archakov, Lipid Peroxidation in Biological Membranes (Nauka, Moscow, 1972) [in Russian].
Yu. A. Vladimirov and E. V. Proskurnina, Usp. Biol. Khim. 49, 341 (2009).
W. Lepeschkin, Science 76, 409 (1932). https://doi.org/10.1126/science.76.1975.409
W. W. Lepeschkin, Science 76, 168 (1932). https://doi.org/10.1126/science.76.1964.168
A. G. Gurvich, Mitogenetic Radiation (Gosmedizdat, Moscow, 1934) [in Russian].
Yu. A. Vladimirov, O. A. Azizova, A. I. Deev, et al., Itogi Nauki Tekh., Ser.: Biofiz. 29 (VINITI, Moscow, 1991).
Yu. A. Vladimirov, E. V. Proskurnina, and D. Yu. Izmailov, Bull. Exp. Biol. Med. 144 (3) 390 (2007).
A. I. Zhuravlev and S. M. Zubkova, Antioxidants: Free Radical Pathology, Aging, 2nd ed. (Belye Al’vy, Moscow, 2014) [in Russian].
A. M. Polimovam M. M. Sozarukova, E. V. Proskurnina, and Yu. A. Vladimirov, in Proc. 2nd Russian Congress of Analysts (Moscow, 2013), p. 276 [in Russian].
Yu. A. Vladimirov and F. F. Litvin, Biofizika 4, 601 (1959).
B. N. Tarusov, A. I. Polivoda, and A. I. Zhuravlev, Biofizika 6, 490 (1961).
L. A. Romodin, Yu. A. Vladimirov, N. P. Lysenko, and E. N. Zarudnaya, Izv. Mezhd. Akad. Agrarn. Obraz., No. 42-1, 112 (2018).
P. O. Volkova, A. V. Alekseev, A. A. Dzhatdoeva, et al., Moscow. Univ. Chem. Bull. 71 (1), 87 (2016).
G. K. Vladimirov, M. M. Sozarukova, and D. Yu. Izmailov, in Science and Practice: New Discoveries, Proc. Int. Conf., Ed. by I. M. Shvets, L. A. Ismagilova, V. A. Gur’eva, and V. A. Sedenko (Kirov, 2015), pp. 717–726.
Yu. A. Vladimirov and A. Ya Potapenko, Physicochemical Basis of Photobiological Processes: A Manual for Students in Medicine and Biology (Vysshaya Shkola, Moscow, 1989) [in Russian].
V. S. Sharov, E. S. Dremina, and Yu. A. Vladimirov, Biofizika 40, 428 (1995).
Yu. A. Vladimirov, M. P. Sherstnev, and T. K. Azimbaev, Biofizika 40, 323 (1995).
Yu. A. Vladimirov, V. S. Sharov, E. S. Driomina, et al., Free Radic. Biol. Med. 18, 739 (1995).
PubChem Database. https://pubchem.ncbi.nlm.nih.gov.
J. Jiang, A. Bakan, A. A. Kapralov, et al., Free Radic. Biol. Med. 71, 221 (2014). https://doi.org/10.1016/j.freeradbiomed.2014.02.029
A. S. Vikulina, A. A. Dzhatdoeva, E. N. Lobichenko, et al., Zh. Anal. Khim. 72, 639 (1987).
Isolation and Analysis of Natural Biologically Active Substances (Tomsk State Univ., Tomsk, 2017) [in Russian].
V. S. Sharov, K. Briviba, and H. Sies, Free Radic. Biol. Med. 21, 833 (1996).
Yu. A. Vladimirov and E. V. Proskurnina, Lectures in Medical Biophysics (Akademkniga, Moscow, 2007) [in Russian].
O. V. Vasiljeva, O. B. Lyubitsky, G. I. Klebanov, and Yu. A. Vladimirov, Membr. Cell Biol. 12, 223 (1998).
A. Mandal, C. L. Hoop, M. DeLucia, et al., Biophys. J. 109, 1873 (2015). https://doi.org/10.1016/j.bpj.2015.09.016
V. E. Kagan, A. Bayir, H. Bayir, et al., Mol. Nutr. Food Res. 53, 104 (2009). https://doi.org/10.1002/mnfr.200700402
V. E. Kagan, G. G. Borisenko, Y. Y. Tyurina, et al., Free Radic. Biol. Med. 37, 1963 (2004). https://doi.org/10.1016/j.freeradbiomed.2004.08.016
L. A. Romodin, E. N. Zarudnaya, and Yu. A. Vladimirov, The Cytochrome c–Cardiolipin Complex: Biological Role and Inhibition by Antioxidants (Pero, Moscow, 2017) [in Russian].
A. Deshpande, S. Nimsadkar, and S. C. Mande, Acta Crystallogr. D. Biol. Crystallogr. 61, 1005 (2005). https://doi.org/10.1107/S0907444905009364
J. N. Rodriguez-Lopez, D. J. Lowe, J. Hernandez-Ruiz, et al., J. Am. Chem. Soc. 123, 11838 (2001).
P. G. Furtmuller, W. Jantschko, M. Zederbauer, et al., Jpn. J. Infect. Dis. 57, S30 (2004).
F. Ito, Y. Sono, and T. Ito, Antioxidants (Basel) 8 (3), 72 (2019). https://doi.org/10.3390/antiox8030072
L. A. Romodin, S. V. Shangin, Yu. A. Vladimirov, et al., Izv. Mezhd. Akad. Agrarn. Obraz., No. 42-1, 118 (2018).
M. J. Cormier and P. M. Prichard, J. Biol. Chem. 243, 4706 (1968).
Funding
This work was supported by the Russian Foundation for Basic Research, grant no. 18-015-00491 “Study of the mechanism of the reactions of formation of free radicals in cell membranes and mitochondria catalyzed by the complex of cytochrome c with anionic lipids (Cyt−AL)”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
All authors of this work declare that they have no conflicts of interest regarding the materials presented in the work.
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain a description of the research performed by the authors with the participation of humans or the use of animals as objects.
Additional information
Abbreviations: CytC-CL—complex of cytochrome c with cardiolipin; CytC-TOCL—complex of cytochrome c with tetraoleylcardiolipin; EES—excited electronic states; EPR—method of electron paramagnetic resonance; C-314—isoquinolysine derivative of coumarin, coumarin-314; C-334—isoquinolysine derivative of coumarin, coumarin-334; C-525—isoquinolysine derivative of coumarin, coumarin-525; ε—molar absorption coefficient.
Rights and permissions
About this article
Cite this article
Romodin, L.A., Vladimirov, Y.A., Shangin, S.V. et al. Isoquinoline Coumarin Derivatives as Chemiluminescence Activators in Reactions of Lipid Peroxidation. BIOPHYSICS 65, 577–586 (2020). https://doi.org/10.1134/S0006350920040181
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006350920040181