Abstract
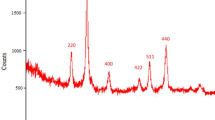
Methionine-coated Fe3O4 nanoparticles, a magnetically reusable and environmentally friendly heterogeneous catalyst, was synthesized. The new catalyst was characterized by FT-IR spectra, XRD, SEM, and EDX analysis and was used to catalyze the cycloaddition of nitriles and sodium azide in DMSO at 120°C to give the corresponding 5-substituted 1H-tetrazoles. Methionine-coated Fe3O4 nanoparticles proved to be highly efficient for this organic reaction. The catalyst can be easily separated and reused several times without loss of activity. The proposed procedure also offers several benefits such as quick reaction, high yields, clean process, low-cost heterogeneous catalyst, low loading of catalyst, and simple operation.








Similar content being viewed by others
REFERENCES
Myznikov, L.V., Hrabalek, A., and Koldobskii, G.I., Chem. Heterocycl. Compd., 2007, vol. 43, p. 1. https://doi.org/10.1007/s10593-007-0001-5
Ostrovskii, V.A., Trifonov, R.E., and Popova, E.A., Russ. Chem. Bull., Int. Ed., 2012, vol. 61, p. 768. https://doi.org/10.1007/s11172-012-0108-4
Wei, C., Bian, M., and Gong, G., Molecules, 2015, vol. 20, p. 5528. https://doi.org/10.3390/molecules20045528
Dolusic, E., Larrieu, P., Moineaux, L., Stroobant, V., Pilotte, L., Colau, D., Pochet, L., Van den Eynde, B.T., Masereel, B., and Wouters, J. J. Med. Chem., 2011, vol. 54, p. 5320. https://doi.org/10.1021/jm2006782
Sarvary, A. and Maleki, A., Mol. Diversity, 2015, vol. 19, p. 189. https://doi.org/10.1007/s11030-014-9553-3
Jung, M.E., Lal, H., and Gatch, M.B., Neurosci. Biobehav. Rev., 2002, vol. 26, p. 429. https://doi.org/10.1016/S0149-7634(02)00010-6
Ornstein, P. L., Arnold, M. B., Allen, N. K., Bleisch, T., Borromeo, P.S., Lugar, C. W., Leander, J. D., Lodge, D., and Schoepp, D.D., J. Med. Chem., 1996, vol. 39, p. 2219. https://doi.org/10.1021/jm950912p
Hiskey, M., Chavez, D.E., Noud, D.C., Son, S.F., Berghout, H.L., Bome, C.A., Proc. Int. Pyrotech. Semin., 2000, vol. 27, p. 3.
Frija, L.M.T., Fausto, R., Loureiro, R.M.S., and Cristiano, M.L.S., J. Mol. Catal. A: Chem., 2009, vol. 305, p. 142. https://doi.org/10.1016/j.molcata.2008.12.007
Jursic, B.S. and Leblanc, B.W., J. Heterocycl. Chem., 1998, vol. 35, p. 405. https://doi.org/10.1002/jhet.5570350224
Hennessy, E.J., Cornebise, M., Gingipalli, L., Grebe, T., Hande, S., Hoesch, V., Huynh, H., Throner, S., Varnes, J., and Wu, Y., Tetrahedron Lett., 2017, vol. 58, p. 1709. https://doi.org/10.1016/j.tetlet.2017.03.056
Gawande, S.D., Raihan, M.J., Zanwar, M.R., Kavala, V., Janreddy, D., Kuo, C., Chen, M., Kuo, T., and Yao, C., Tetrahedron, 2013, vol. 69, p. 1841. https://doi.org/10.1016/j.tet.2012.12.062
Jin, T., Kitahara, F., Kamijo, S., and Yamamoto, Y., Tetrahedron Lett., 2008, vol. 49, p. 2824. https://doi.org/10.1016/j.tetlet.2008.02.115
Kantam, M.L., Shiva Kumar, K.B., and Sridhar, C., Adv. Synth. Catal., 2005, vol. 347, p. 1212. https://doi.org/10.1002/adsc.200505011
Akula, R.K., Adimulam, C.S., Gangaram, S., Kengiri, R., Banda, N., and Pamulaparthy, S.R., Lett. Org. Chem., 2014, vol. 11, p. 440. https://doi.org/10.2174/1570178611666140210213157
Akhlaghinia, B. and Rezazadeh, S., J. Braz. Chem. Soc., 2012, vol. 23, p. 2197. https://doi.org/10.1590/S0103-50532013005000005
Bonnamour, J. and Bolm, C., Chem. Eur. J., 2009, vol. 15, p. 4543. https://doi.org/10.1002/chem.200900169
Zarghani, M. and Akhlaghinia, B., RSC Adv., 2016, vol. 6, p. 31850. https://doi.org/10.1039/C6RA07252F
Abdollahi-Alibeika, M. and Moaddeli, A., New J. Chem., 2014, vol. 38, p. 3102. https://doi.org/10.1039/C3NJ01149F
Guggilapu, S.D., Prajapti, S.K., Gupta, A.N.K.K., and Babu, B.N., Synlett, 2016, vol. 27, p. 1241. https://doi.org/10.1055/s-0035-1561559
Mani, A.K., Singh, S.K., and Wasthi, A., Tetrahedron Lett., 2014, vol. 55, p. 1879. https://doi.org/10.1016/j.tetlet.2014.01.117
Bayat, N.Y., Habibi, D., and Moshaee, S., Tetrahedron Lett., 2009, vol. 50, p. 4435. https://doi.org/10.1016/j.tetlet.2009.05.048
Vorona, S., Artamonova, T., Zevatskii, Y., and Myznikov, L., Synthesis, 2014, vol. 46, p. 781. https://doi.org/10.1055/s-0033-1340616
Gowd, M.R.M.B. and Pasha, M.A., J. Chem. Sci., 2011, vol. 123, p. 75. https://doi.org/10.1007/s12039-011-0065-8
Prajapti, S.K., Nagarsenkar, A., and Babu, B.N., Tetrahedron Lett., 2014, vol. 55, p. 3507. https://doi.org/10.1016/j.tetlet.2014.04.089
Ishihara, K., Kawashima, M., Shioiri, T., and Matsugi, M., Synlett, 2016, vol. 27, p. 2225. https://doi.org/10.1055/s-0035-1561668
He, J., Li, B., Chen, F., Xu, Z., and Yin, G., J. Mol. Catal. A: Chem., 2009, vol. 304, p. 135. https://doi.org/10.1016/j.molcata.2009.01.037
Meshram, G.A., Deshpande, S.S., Wagh, P.A., and Vala, V.A., Tetrahedron Lett., 2014, vol. 55, p. 3555. https://doi.org/10.1016/j.tetlet.2014.04.101
Aridoss, G. and Laali, K.K., Eur. J. Org. Chem., 2011, vol. 2011, p. 6343. https://doi.org/10.1002/ejoc.201100957
Das, B., Reddy, C.R., Kumar, D.N., Krishnaiah, M., and Narender, R., Synlett, 2010, vol. 2010, no. 3, p. 391. https://doi.org/10.1055/s-0029-1219150
Lang, L., Li, B., Liu, W., Li, J., Xu, Z., and Yin, G., Chem. Commun., 2010, vol. 46, p. 448. https://doi.org/10.1039/B912284B
Rama, V., Kanagaraj, K., and Pitchumani, K., J. Org. Chem., 2011, vol. 76, p. 9090. https://doi.org/10.1021/jo201261
Ghodsinia, S.S.E. and Akhlaghinia, B., RSC Adv., 2015, vol. 5, p. 49849. https://doi.org/10.1039/C5RA08147E
Khan, K.M., Fatima, I., Saad, S.M., Taha, M., and Voelter, W., Tetrahedron Lett., 2016, vol. 57, p. 523. https://doi.org/10.1016/j.tetlet.2015.12.067
Bosch, L. and Vilarrasa, J., Angew. Chem., Int. Ed., 2007, vol. 46, p. 3926. https://doi.org/10.1002/anie.200605095
Patil, V.S., Nandre, K.P., Borse, A.U., and Bhosale, S.V., J. Chem., 2012, vol. 9, article ID 615891. https://doi.org/10.1155/2012/615891
Marvi, O., Alizadeh, A., and Zarrahi, S., Bull. Korean Chem. Soc., 2011, vol. 32, p. 4001. https://doi.org/10.5012/bkcs.2011.32.11.4001
Cantillo, D., Gutmann, B., and Kappe, C.O., J. Am. Chem. Soc., 2011, vol. 133, p. 4465. https://doi.org/10.1021/ja109700b
Myznikov, L.V., Efimova, Yu.A., Artamonova, T.V., and Koldobskii, G.I., Russ. J. Org. Chem., 2011, vol. 47, p. 728. https://doi.org/10.1134/s1070428011050125
Karimian, A., Mohammadzadeh Kakhki, R., and Kargar Beidokhti, H., J. Chin. Chem. Soc., 2017, vol. 64, p. 1316. https://doi.org/10.1002/jccs.201700060
Karimian, A., J. Heterocycl. Chem., 2018, vol. 55, p. 645. https://doi.org/10.1002/jhet.3082
Karimian, A. and Karimian, H., J. Chem. Res., 2017, vol. 41, p. 406. https://doi.org/10.3184/174751917X14967701767049
Karimian, A., Eshghi, H., Bakavoli, M., Shiri, A., Saadatmandzadeh, M., Asghari, T., and Moradi, H., J. Iran. Chem. Soc., 2015, vol. 12, p. 1501. https://doi.org/10.1007/s13738-015-0620-1
Karimian, A., Eshghi, H., Bakavoli, M., and Shiri, A., J. Heterocycl. Chem., 2015, vol. 54, p. 151. https://doi.org/10.1002/jhet.2556
Asgari, S., Fakhari, Z., and Berijani, S., J. Nanostruct., 2014, vol. 4, p. 55. https://doi.org/10.7508/jns.2014.01.007
Li, Y.-S., Church, J.S., and Woodhead, A.L., J. Magn. Mater., 2012, vol. 324, p. 1543. https://doi.org/10.1016/j.jmmm.2011.11.065
Gupta, P.K., Tiwari, S., Khan, Z.H., and Solanki, P.R., J. Mater. Chem. B, 2017, vol. 5, p. 2019. https://doi.org/10.1039/C6TB02594C
JCPDS Data Card, International Center of Diffraction Data. Swarthmore, PA. 1988.
Park, J.Y., Choi, E.S., Baek, M.J., and Lee, G.H., Mater. Lett., 2009, vol. 63, p. 379. https://doi.org/10.1016/j.matlet.2008.10.057
Namvar-Mahboub, M., Khodeir, E., and Karimian, A., Res. Chem. Intermed., 2018, vol. 44, p. 6877. https://doi.org/10.1007/s11164-018-3527-5
Du, Z., Si, C., Li, Y., Wang, Y., and Lu, J., Int. J. Mol. Sci., 2012, vol. 13, p. 4696. https://doi.org/10.3390/ijms13044696
Rostamizadeh, S., Ghaieni, H., Aryan, R., and Amani, A., Chin. Chem. Lett., 2009, vol. 20, p. 1311. https://doi.org/10.1016/j.cclet.2009.06.020
Paudel, S., Min, X., Achary, S., Khadka, D.B., Yoon, G., Kim, K.M., and Cheon, S.H., Bioorg. Med. Chem., 2017, vol. 25, p. 5278. https://doi.org/10.1016/j.bmc.2017.07.046
Swapnil, A.P. and Dipak, S.D., Synth. Commun., 2017, vol. 47, p. 779. https://doi.org/10.1080/00397911.2017.1285033
Razavi, N. and Akhlaghinia, B., RSC Adv., 2015, vol. 5, p. 12372. https://doi.org/10.1039/C4RA15148H
Kantam, M.L., Shiva Kumar, K.B., and Phani Raja, K.J., J. Mol. Catal. A: Chem., 2006, vol. 247, p. 186. https://doi.org/10.1016/j.molcata.2005.11.046
Paudel, S., Min, X., Achary, S., Khadka, D.B., Yoon, G., Kim, K.M., and Cheon, S.H., Bioorg. Med. Chem., 2017, vol. 25, p. 5278. https://doi.org/10.1016/j.bmc.2017.07.046
Taghavi, F., Gholizadeh, M., Saljooghi, A.S., and Ramezani, M., Med. Chem. Commun., 2017, vol. 8, p. 1953. https://doi.org/10.1039/C7MD00302A
Wang, H., Zhao, W., Du, J., Wei, F., Chen, Q., and Wang, X., Appl. Organomet. Chem., 2019, vol. 33, p. 5132. https://doi.org/10.1002/aoc.5132
ACKNOWLEDGMENTS
The authors gratefully acknowledge the partial support of this study by the University of Gonabad Research Council. This research did not receive any specific funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Karimian, A., Namvar-Mhaboub, M. & Abbasi, R. Methionine-Coated Fe3O4 Nanoparticles: An Efficient and Reusable Nanomagnetic Catalyst for the Synthesis of 5-Substituted 1H-Tetrazoles. Russ J Org Chem 56, 1646–1653 (2020). https://doi.org/10.1134/S1070428020090237
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428020090237