Abstract
Purpose
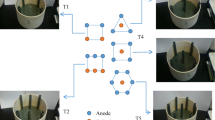
Electrokinetic (EK) soil remediation is significantly affected by the electrode configurations. Therefore, this study was conducted to compare the performance of one-dimensional (1D) and two-dimensional (2D) arrays with respect to the remediation of heavy metal-contaminated soils.
Materials and methods
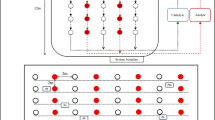
A series of laboratory-scale experiments were carried out using two types of soils: kaolinite soil (KS) artificially spiked with heavy metals (Cd, Cu, Pb, Ni, and Zn) and smelter soil (SS) contaminated with Cu and Pb, obtained near a closed smelting factory. First, stepwise experiments on the KS were conducted to compare the EK performance between the 1D and 2D electrode arrays. More specifically, the effect of the number of anode–cathode pairs was investigated in the experiments on the 1D electrode configuration, and trigonal and hexagonal arrangements were compared in the 2D EK experiments. Additionally, the performance of the trigonal array was evaluated according to changes in the anode positions, to switch the areas of effective and ineffective electric field.
Results and discussion
The removal efficiencies of the 1D electrode configuration with four anode–cathode pairs (eight electrodes in total) were 69.1% for Cd, 69.2% for Cu, 74.7% for Ni, 28.3% for Pb, and 71.3% for Zn. The removal efficiencies of the 2D hexagonal electrode array, with a similar number of electrodes (six anodes and one cathode for seven electrodes in total), were 79.8% (Cd), 82.6% (Cu), 83.7% (Ni), 34.3% (Pb), and 81.1% (Zn). The total electric power consumptions for the two types of electrode configurations were investigated to be 15.6 and 22.0 kWh/ton, respectively. Despite the increase in electric power consumption in the 2D electrode configuration, it was more efficient than the 1D configuration due to the increased area of effective (active) electric field and subsequently higher removal efficiency. As a result of changing the positions of anodes in the trigonal 2D arrangement, the removal efficiencies of Cu and Pb were increased to a level similar to that obtained with the hexagonal configuration.
Conclusions
This study demonstrated that a 2D electrode configuration increased the EK removal efficiency of contaminants compared with a 1D array. However, cost-effectiveness should be taken into account when optimizing electrode design. Furthermore, the performance of the trigonal 2D EK process was improved by changing the positions of anodes, as a result of the increase in the removal efficiency and decrease in the electrode cost and energy consumption.










Similar content being viewed by others
References
Agnew K, Cundy AB, Hopkinson L, Croudace IW, Warwick PE, Purdie P (2011) Electrokinetic remediation of plutonium-contaminated nuclear site wastes: results from a pilot-scale on-site trial. J Hazar Mater 186:2171–2187
Alshawabkeh AN (2009) Electrokinetic soil remediation: challenges and opportunities. Sep Sci Technol 44:2171–2187
Alshawabkeh AN, Yeung AT, Bricka MR (1999) Practical aspects of in-situ electrokinetic extraction. J Environ Eng 125:27–35
Cuevas O, Herrada RA, Corona JL, Olvera MG, Sepúlveda-Guzmán S, Sirés I (2016) Assessment of IrO2-Ta2O5|Ti electrodes for the electrokinetic treatment of hydrocarbon-contaminated soil using different electrode arrays. Electrochim Acta 208:282–287
Farrah H, Pickering WF (1977) The sorption of lead and cadmium species by clay minerals. Aust J Chem 30:1417–1422
Ferrucci A, Vocciante M, Bagatin R, Ferro S (2017) Electrokinetic remediation of soils contaminated with potentially toxic metals: dedicated analytical tools for assessing the contamination baseline in a complex scenario. J Environ Manag 203:1163–1168
Figueroa A, Cameselle C, Gouveia S, Hansen HK (2016) Electrokinetic treatment of an agricultural soil contaminated with heavy metals. J Environ Sci Health A Tox Hazard Subst Environ Eng 51:691–700
Hamed J, Acar YB, Gale RJ (1991) Pb(II) removal from kaolinite by electrokinetics. J Geotech Eng 117:241–271
Jo SU, Shin YJ, Yang JS, Moon DH, Koutsospyros A, Baek K (2015) Enhanced electrokinetic transport of sulfate in saline soil. Water Air Soil Pollut 226:199
Kim WS, Jeon EK, Jung JM, Jung HB, Ko SH, Seo CI, Baek K (2014) Field application of electrokinetic remediation for multi-metal contaminated paddy soil using two-dimensional electrode configuration. Environ Sci Pollut Res 21:4482–4491
Kim KY, Kim SO (2002) Electrokinetic soil processing for energy from waste. In: Grover VI, Grover VK, Hogland W (eds) Recovering energy from waste: various aspects. Scientific Publishers, Inc., Enfield, pp 107–140
Kim SO, Kim KY, Stüben D (2002) Evaluation of electrokinetic removal of heavy metals from tailing soils. J Environ Eng 128:705–715
Kim SO, Lee KY, Kim KY (2015) Fundamentals of electrokinetics. In: Bundschuh J, Holländer HM, Ma LQ (eds) In-situ remediation of arsenic-contaminated sites. CRC Press/Balkema, EH Leiden, pp 87–113
Liu F (2013) Non-uniform electrokinetic removal of heavy metals from contaminated soil with permeable reactive composite electrodes. Appl Mechan Mater 206–261:1145–1150
Liu S, Li W, Song W, Guo M (2018) Review of remediation techniques for heavy metal-contaminated soils: principles and applicability. Sci Total Environ 633:206–219
Mao X, Shao X, Zhang Z, Han F (2018) Mechanimization of enhanced electro-kinetic remediation on 137Cs contaminated kaolin soils: a semi-pilot study based on experimental and modeling methodology. Electrochim Acta 284:38–51
Méndez E, Pérez M, Romero O, Beltrán ED, Castro S, Corona JL, Corona A, Cuevas MC, Bustos E (2012) Effects of electrode material on the efficiency of hydrocarbon removal by an electrokinetic remediation process. Electrochim Acta 86:148–156
Page MM, Page C (2002) Electroremediation of contaminated soil. J Environ Eng 128:208–219
Schwertman U, Taylor RM (1989) Iron oxides. Minerals in soil environments, 2nd ed., SSSA Book Series No. 1, Soil Science of America, Madison, pp 379–427
Sivapullaiah PV, Sriakantappa B, Prakash N, Suma BN (2015) Electrokinetic removal of heavy metals from soil. J Electrochem Sci Eng 5:47–65
Song Y, Ammami MT, Benamar A, Mezazigh S, Wang H (2016) Effect of EDTA, EDDS, NTA and citric acid on electrokinetic remediation of As, Cd, Cr, Cu, Ni, Pb, and Zn contaminated dredged marine sediment. Environ Sci Pollut Res Int 21:3126–3133
Sun Y, Gao K, Zhang Y, Zou H (2017) Remediation of persistent organic pollutant-contaminated soil using biosurfactant-enhanced electrokinetics coupled with a zero-valent iron/activated carbon permeable reactive barrier. Environ Sci Pollut Res 24:28142–28151
Virkuytyte J, Sillanpää M, Latostenmaa R (2002) Electrokinetic soil remediation-critical overview. Sci Total Environ 289:97–121
Yoo JC, Yang JS, Jeon EK, Baek K (2015) Enhanced-electrokinetic extraction of heavy metals from dredged harbor sediment. Environ Sci Pollut Res 22:9912–9921
Zhang T, Zou H, Ji M, Li X, Li L, Tang T (2014) Enhanced electrokinetic remediation of lead-contaminated soil by complexing agents and approaching anodes. Environ Sci Pollut Res Int 21:3126–3133
Zhou H, Xu J, Lv S, Liu Z, Liu W (2020) Removal of cadmium in contaminated kaolin by new-style electrokinetic remediation using array electrodes coupled with permeable reactive barrier. Sep Purif Technol 239:11654
Funding
This research was supported by Korea Environment Industry and Technology Institute (KEITI) through the Subsurface Environment Management (SEM) Project funded by Korea Ministry of Environment (MOE) (grant number: 2018002440002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Daniel Alessi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1.57 mb)
Rights and permissions
About this article
Cite this article
Kim, SO., Jeong, J.Y., Lee, WC. et al. Electrokinetic remediation of heavy metal-contaminated soils: performance comparison between one- and two-dimensional electrode configurations. J Soils Sediments 21, 2755–2769 (2021). https://doi.org/10.1007/s11368-020-02803-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-020-02803-z