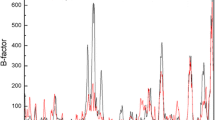
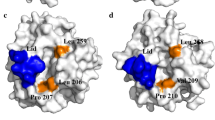
Abstract
To improve the thermostability of the lipase (r27RCL) from Rhizopus chinensis through rational design, a newly introduced buried disulfide bond F223C/G247C was proved to be beneficial to thermostability. Interestingly, F223C/G247C was also found to improve the alkali tolerance of the lipase. Subsequently, six other thermostabilizing mutations from our previous work were integrated into the mutant F223C/G247C, leading to a thermo-alkali-stable mutant m32. Compared to the wild-type lipase, the associative effect of the beneficial mutations showed significant improvements on the thermostability of m32, with a 74.7-fold increase in half-life at 60 °C, a 21.2 °C higher \(T_{50}^{30}\) value and a 10 °C elevation in optimum temperature. The mutated m32 was also found stable at pH 9.0–10.0. Furthermore, the molecular dynamics simulations of m32 indicated that its rigidity was enhanced due to the decreased solvent-accessible surface area, a newly formed salt bridge, and the increased ΔΔG values.








Similar content being viewed by others
References
Hasan F, Shah AA, Hameed A (2006) Industrial application of microbial lipase. Enzyme Microb Tech 39:235–251
Yu X, Xu Y, Xiao R (2016) Lipases from the genus Rhizopus: characteristics, expression, protein engineering and application. Prog Lipid Res 64:57–68
Kamiya N, Ogawa T, Nagamune T (2001) Enhancement of apparent thermostability of lipase from Rhizopus sp. by the treatment with a microbial transglutaminase. Biotechnol Lett 23:1629–1632
Ariaeenejad S, Maleki M, Hosseini E, Kavousi K, Moosavi-Movahedi AA, Salekdeh GH (2019) Mining of camel rumen metagenome to identify novel alkali-thermostable xylanase capable of enhancing the recalcitrant lignocellulosic biomass conversion. Bioresource Technol 281:343–350
Chirstopher LP, Zambare VP, Zambare A, Kumar H, Malek L (2015) A thermos-alkaline lipase from a new thermophile Geobacillus thermodenitrificans AV-5 with potential application in biodiesel production. J Chem Technol Biotechnol 90:2007–2016
Flory PJ (1956) Theory of elastic mechanisms in fibrous proteins. J Am Chem Soc 78:5222–5235
Masazumi M, Giovanni S, Brian WM (1989) Substantial increase of protein stability by multiple disulfide bonds. Nature 342:291–293
Pace CN, Grimsley GR, Thomson JA, Barnett BJ (1988) Conformational stability and activity of ribonuclease T1 with zero, one, and two intact disulfide bonds. J Biol Chem 263:11820–11825
Tidor B, Karplus M (1993) The contribution of cross-links to protein stability: a normal mode analysis of the configurational entropy of the native state. Proteins 15:71–79
Melnik BS, Povarnitsyna TV, Glukhov AS, Melnik TN, Uversky VN, Sarma RH (2012) SS-stabilizing proteins rationally: intrinsic disorder-based design of stabilizing disulfide bridges in GFP. J Biomol Struct Dyn 29:815–824
Dani VS, Ramakrishnan C, Varadarajan R (2003) MODIP revisited: re-evaluation and refinement of an automated procedure for modeling of disulfide bonds in proteins. Protein Eng 16:187–193
Craig DB, Dombkowski AA (2013) Disulfide by Design 2.0: a web-based tool for disulfide engineering in proteins. BMC Bioinformatics 14:346–352
Xu Y, Wang D, Mu XQ, Zhao GA, Zhang KC (2002) Biosynthesis of ethyl esters of short-chain fatty acids using whole-cell lipase from Rhizopus Chinesis CCTCCM201021 in non-aqueous phase. J Mol Catal B-Enzym 18:29–37
Yu X, Wang L, Xu Y (2009) Rhizopus chinensis lipase: Gene cloning, expression in Pichia pastoris and properties. J Mol Catal B-Enzym 57:304–311
Yu XW, Wang R, Zhang M, Xu Y, Xiao R (2012) Enhanced thermostability of a Rhizopus chinensis lipase by in vivo recombination in Pichia pastoris. Microb Cell Fact 11:102–112
Wang R, Yu XW, Xu Y (2018) Rationale design of disulfide bond in Rhizopus chinensis lipase to improve thermostability. Microbiol China 11:2311–2319
Wang R, Wang S, Xu Y, Yu XW (2020) Enhancing the thermostability of Rhizopus chinensis lipase by rational design and MD simulations. Int J Biol Macromol 160:1189–1200
Zhang M, Yu X, Swapna GV, Xiao R, Xu Y (2018) G. Montelione, Backbone and Ile-δ1, Leu, Val methyl chemical shift assignments for Rhizopus microsporus var. chinensis lipase. Bio NMR Assigm 12:63–68
Lee D, Koh Y, Kim K, Kim B, Choi H, Kim D, Suhartono MT, Pyun Y (1999) Isolation and characterization of a thermophilic lipase from Bacillus thermoleovorans ID-1. FEMS Microbiol Lett 179:393–400
Zorn H, Li QX (2017) Trends in food enzymology. J Agric Food Chem 65:4–5
Waterhouse A, Bertoni M, Bienert S et al (2018) SWISS-MODEL: homology modeling of protein structures and complexes. Nucleic Acids Res 46:W296–W303
Abraham MJ, Murtola T, Schulz R, Pall S, Smith JC, Hess B, Lindahl E (2015) GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX1 2:19–25
Schmid N, Elchenberger AP, Choutko A, Riniker S, Winger M, Mark AE, van Gunsteren WF (2011) Definition and testing of the GROMOS force-field versions 54A7 and 54B7. Eur Biophys J 40:843–856
Essmann U, Perera L, Berkowitz ML, Darden T, Lee H (1995) A smooth particle mesh Ewald method. J Chem Phys 103:8577–8593
Darden T, York D, Pedersen L (1993) Particle mesh Ewald: an N⋅log (N) method for Ewald sums in large systems. J Chem Phys B 98:98–110
Hess B, Bekker H, Berendsen HJC, Fraaije JGEM (1998) LINCS: a linear constraint solver for molecular simulations. J Comput Chem 18:1463–1472
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38
Eijsink VGH, Bjørk A, Gåseidnes S, Sirevåg R, Synstad B, van den Burg B, Vriend G (2004) Rational engineering of enzyme stability. J Biotechnol 113:105–120
Johannes TW, Woodyer RD, Zhao HM (2005) Directed evolution of a thermostable phosphite dehydrogenase for NAD(P)H regeneration. Appl Environ Microb 71:5728–5734
Pjura PE, Matsumura M, Wozniak JA, Matthews BW (1990) Structure of a thermostable disulfide-bridge mutant of phage T4 lysozyme shows that an engineered cross-link in a flexible region does not increase the rigidity of the folded protein. Biochemistry 29:2592–2598
Jacobson RH, Matsumura M, Faber HR, Matthews BW (1992) Structure of a stabilizing disulfide bridge mutant that closes the active-site cleft of T4 lysozyme. Protein Sci 1:46–57
Wakarchuk WW, Sung WL, Campbell RL, Cunningham A, Watson DC, Yaguchi M (1994) Thermostabilization of the Bacillus circulans xylanase by the introduction of disulfide bonds. Protein Eng 7:1379–1386
Clarke J, Henrick K, Fersht AR (1995) Disulfide mutants of barnase, I: changes in stability and structure assessed by biophysical methods and X-ray crystallography. J Mol Biol 253:493–504
Tanghe M, Danneels B, Last M, Beerens K, Stals I, Desmet T (2017) Disulfide bridges as essential elements for the thermostability of lytic polysaccharide monooxygenase LPMO10C from Streptomyces coelicolor. Protein Eng Des Sel 30:401–408
Katz BA, Kossiakoff A (1986) The crystallographically determined structures of atypical strained disulfides engineered into subtilisin. J Biol Chem 261:15480–15485
Mitchinson C, Wells JA (1989) Protein engineering of disulfide bonds in subtilisin BPN’: enhanced stabili zation through the introduction of two cysteines to form a disulfide bond. Biochemistry 28:4807–4815
Reetz MT, Carballeira JD, Vogel A (2006) Iterative saturation mutagenesis on the basis of B-factors as a strategy for increasing protein thermostability. Angew Chem Int Edit 45:7745–7751
Zhang X, Yang G, Zhang Y, Xie Y, Withers SG, Feng Y (2016) A general and efficient strategy for generating the stable enzymes. Sci Rep 6:33797
Teilum K, Olsen JG, Kragelund BB (2009) Functional aspects of protein flexibility. Cell Mol Life Sci 66:2231–2247
Pezeshgi Modarres H, Dorokhov BD, Popov VO, Ravin NV, Skryabin KG, Dal Peraro M (2015) Understanding and engineering thermostability in DNA Ligase from Thermococcus sp 1519. Biochemistry 54:3076–3085
Liu Z, Lemmonds S, Huang J, Tyagi M, Hong L, Jain N (2018) Entropic contribution to enhanced thermal stability in the thermostable P450 CYP119. PNAS 115:E10049–E10058
Badieyan S, Bevan DR, Zhang C (2012) Study and design of stability in GH5 cellulases. Biotech Bioeng 109:31–44
Anderson DE, Becktel WJ, Dahlquist FW (1990) pH-induced denaturation of proteins: a single salt bridge contributes 3–5 kcal/mol to the free energy of folding of T4 lysozyme. Biochemistry 29:2403–2408
Donald JE, Kulp DW, DeGrade WF (2011) Salt bridge: geometrically specific, designable interactions. Proteins 79:898–915
Acknowledgements
Financial support from the National Natural Science Foundation of China (31671799), the Six Talent Peaks Project in Jiangsu Province (NY-010), the High-end Foreign Experts Recruitment Program (GDT20153200044), the National First-Class Discipline Program of Light Industry Technology and Engineering (LITE2018-09), and the 111 Project (111-2-06) are greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, R., Wang, S., Xu, Y. et al. Engineering of a thermo-alkali-stable lipase from Rhizopus chinensis by rational design of a buried disulfide bond and combinatorial mutagenesis. J Ind Microbiol Biotechnol 47, 1019–1030 (2020). https://doi.org/10.1007/s10295-020-02324-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-020-02324-1