Abstract
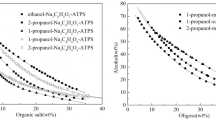
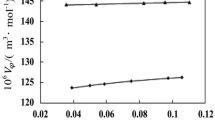
Imidazolium-based ionic liquids 1-hexyl-3-methylimidazolium hexaflorophosphate, and 1-hexyl-3-methylimidazolium tetrafloroborate were used to determine pH dependent-ionic forms of Valine and Aspartic acid in aqueous solutions. For this purpose, the partitioning behaviors of both compounds between the aqueous phase with different pH values and ionic liquid phase were investigated. The results revealed that due to having different pH-dependent ionic forms, each compound showed different partitioning behavior. Partitioning behaviors of Valine and Aspartic acid depend strongly to aqueous solution acidity and chemical structures of ionic liquids. Water content of ionic liquid phase and charge density of anionic and cationic constituents of ionic liquids are effective parameters for extraction of studied amino acids into ionic liquid phase. This study was considered as a basis for introducing a green technique to realize the real ionic species of different amphoteric compounds such as amino acids in the aqueous solutions.


Similar content being viewed by others
References
Absalan G, Akhond M, Sheikhian L (2008) Extraction and high performance liquid chromatographic determination of 3-indole butyric acid in pea plants by using imidazolium-based ionic liquids as extractant. Talanta 77:407–411
Absalan G, Akhond M, Sheikhian L (2010) Partitioning of acidic, basic and neutral amino acids into imidazolium-based ionic liquids. Amino Acids 39:167–174
Adams EQ (1916) Relations between the constants of basic acids and of amphoteric electorolytes. J Am Chem Soc 38:1503–1510
Anderson JL, Ding J, Welton T, Armstrong DW (2002) Characterizing ionic liquids on the basis of multiple solvation interactions. J Am Chem Soc 124:14247–14254
Bretti C, Giuffrè O, Lando G, Sammartano S (2016) Modeling solubility and acid-base properties of some amino acids in aqueous NaCl and (CH3)4NCl aqueous solutions at different ionic strengths and temperatures. SpringerPlus 5:928–949
Cammarata L, Kazarian SG, Salter PA, Welton T (2001) Molecular states of water in room temperature ionic liquids. Phys Chem Chem Phys 3:5192–5200
Carda-Broch S, Berthod A, Armstrong DW (2003) Solvent properties of the 1-butyl-3-methylimidazolium hexafluorophosphate ionic liquid. Anal Bioanal Chem 375:191–199
Chauque S, Oliva FY, Cámara OR, Torresi RM (2018) Use of poly[ionic liquid] as a conductive binder in lithium ion batteries. J Solid State Electrochem 22:3589–3596
Fam W, Mansouri J, Li H, Hou J, Chen V (2019) Effect of inorganic salt blending on the CO2 separation performance and morphology of pebax1657/ionic liquid gel membranes. Ind Eng Chem Res 58:3304–3313
Freire MG, Santos LMNBF, Fernandes AM, Coutinho JAP, Marrucho IM (2007) An overview of the mutual solubilities of water-imidazolium-based ionic liquids systems. Fluid Phase Equilib 26:449–454
Harris LJ (1930) Proof of the zwitterion constitution of the amino-acid molecule. Biochem J 24:1080–1097
Jia H, Kloepsch R, He X, Evertz M, Nowak S, Li J, Winter M, Placke T (2016) Nanostructured ZnFe2O4 as anode material for lithium ion batteries: ionic liquid-assisted synthesis and performance evaluation with special emphasis on comparative metal dissolution. Acta Chim Slov 63:470–483
Koel M (2009) Ionic liquids in chemical analysis. Taylor & Francis, Boca Raton
Kong L, Chen Q, Shen X, Zhu G, Zhu J (2018) Ionic liquid directed construction of foam-like mesoporous boron-doped graphitic carbon nitride electrode for high-performance supercapacitor. J Colloid Interface Sci 532:261–271
Marchese R, Gandori R, Carloni P, Raugei S (2010) On the zwitterionic nature of gas-phase peptides and protein ions. PLoS Comput Biol 6:1–11
Michaelis L (1926) Hydrogen ion concentration, Williams & Wilkins Co, 2nd edn. London
Noda A, Hayamizu K, Watanabe M (2001) Pulsed-gradient spin−echo 1H and 19F NMR ionic diffusion coefficient, viscosity, and ionic conductivity of non-chloroaluminate room-temperature ionic liquids. J Phys Chem B 105:4603–4610
Ortiz-Martínez VM, Ortiz A, Fernández-Stefanutoc V, Tojo E, Colpaert M, Améduri B, Ortiz I (2019) Fuel cell electrolyte membranes based on copolymers of protic ionic liquid [HSO3-BVIm][TfO] with MMA and hPFSVE. Polymer 179:121583–121593
Price WD, Jockusch RA, Williams ER (1997) Is arginine a zwitterion in the gas phase? J Am Chem Soc 119:11988–11989
Rzelewska-Piekut M, Regel-Rosocka M (2019) Separation of Pt(IV), Pd(II), Ru(III) and Rh(III) from model chloride solutions by liquid–liquid extraction with phosphonium ionic liquids. Sep Purif Technol 212:791–801
Sheikhian L, Bina S (2016) Simultaneous extraction and HPLC determination of 3-indole butyricacid and 3- indole acetic acid in pea plant by using ionicliquid-modified silica as sorbent. J Chromatogr B 1008:34–43
Shekaari H, Jebali F (2010) Densities and electrical conductances of amino acids + ionic liquid ([HMIm]Br) + H2O mixtures at 298.15 K. Fluid Phase Equilib 295:68–75
Sun SW, Lin YC, Weng YM, Chen MJ (2006) Efficiency improvements on Ninhydrin method for amino acid quantification. J Food Compos Anal 19:112–117
Tsunekawa H, Narumi A, Sano M, Hiwara A, Fujita M, Yokoyama H (2003) Solvation and ion association studies of LiBF4-propylenecarbonate and LiBF4-propylenecarbonate-trimethyl phosphate solutions. J Phys Chem B 107:10962–10966
Walton T (1999) Room-temperature ionic liquids solvents for synthesis and catalysis. Chem Rev 99:2071–2084
Wang J, Pei Y, Zhao Y, Hu Z (2005) Recovery of amino acids by imidazolium based ionic liquids from aqueous media. Green Chem 7:196–202
Weiss E, Dutta B, Kirschning A, Abu-Reziq R (2014) BMIm-PF6@SiO2 microcapsules: particulated ionic liquid as a new material for the heterogenization of catalysts. Chem Mater 26:4781–4787
Wu R, McMahon TB (2005) An investigation of the ion-molecule interactions of protonated glycine with ammonia by high pressure mass spectrometry and ab initio calculations. Can J Chem 83:1978–1993
Wu R, McMahon TB (2007) Infrared multiple photon dissociation spectra of proline and glycine proton-bound homodimers. Evidence for zwitterionic structure. J Am Chem 129:4864–4865
Wu R, McMahon TB (2008) Stabilization of zwitterionic structures of amino acids (Gly, Ala, Val, Leu, Ile, Ser and Pro) by ammonium ions in the gas phase. J Am Chem 130:3065–3078
Wu R, Marta RA, Martens JK, Eldridge KR, McMahon TB (2011) Experimental and theoretical investigation of the proton-bound dimer of lysine. J Am Soc Mass Spectrom 22:1651–1659
Wyttenbach T, Witt M, Bowers MT (2000) On the stability of amino acid zwitterions in the gas phase: the influence of derivatization, proton affinity, and alkali ion addition. J Am Chem Soc 122:3458–3464
Yaminsky VV, Vogler EA (2001) Hydrophobic hydration. Curr Opin Colloid Interface Sci 6(4):342–349
Yang Z, Li Q, Yang G (2016) Zwitterionic structures: from physicochemical properties toward computer-aided drug designs. Future Med Chem 8:2245–2262
Acknowledgements
The author is grateful to Young Researchers and Elite Club, Kazerun Branch, Islamic Azad University for financial support of this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sheikhian, L. Characterization of pH Dependent-Charged Structures of Valine and Aspartic Acid Through Their Partitioning into Imidazolium-Based Ionic Liquids. Iran J Sci Technol Trans Sci 45, 155–161 (2021). https://doi.org/10.1007/s40995-020-00987-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40995-020-00987-0