Exogenous Nitric Oxide Delays Plant Regeneration from Protoplast and Protonema Development in Physcomitrella patens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Exogenous NO Treatments
2.3. Protoplast Isolation and NO Treatment
2.4. Plant Growth and Development Estimation
3. Results and Discussion
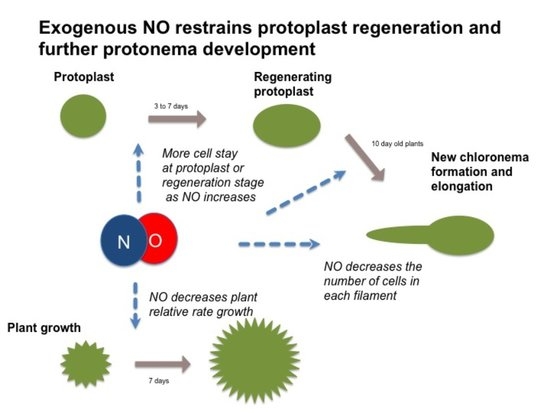
3.1. NO Donor Decreases Relative Growth of P. patens
3.2. NO Donor Delays the Regeneration Process and Chloronema Initiation
3.3. NO Donors also Inhibited Cell Cycle in Regenerated Plants
Author Contributions
Funding
Conflicts of Interest
References
- Besson-Bard, A.; Pugin, A.; Wendehenne, D. New Insights into Nitric Oxide Signaling in Plants. Annu. Rev. Plant Biol. 2008, 59, 21–39. [Google Scholar] [CrossRef]
- Moreau, M.; Lindermayr, C.; Durner, J.; Klessig, D.F. NO synthesis and signaling in plants—Where do we stand? Physiol. Plant. 2010, 138, 372–383. [Google Scholar] [CrossRef]
- Astier, J.; Gross, I.; Durner, J. Nitric oxide production in plants: An update. J. Exp. Bot. 2017, 69, 3401–3411. [Google Scholar] [CrossRef]
- Yamasaki, H.; Sakihama, Y. Simultaneous production of nitric oxide and peroxynitrite by plant nitrate reductase: In vitro evidence for the NR-dependent formation of active nitrogen species. FEBS Lett. 2000, 468, 89–92. [Google Scholar] [CrossRef] [Green Version]
- Tun, N.; Santa-Catarina, C.; Begum, T.; Silveira, V.; Handro, W.; Floh, E.I.S.; Scherer, G.F.E. Polyamines Induce Rapid Biosynthesis of Nitric Oxide (NO) in Arabidopsis thaliana Seedlings. Plant Cell Physiol. 2006, 47, 346–354. [Google Scholar] [CrossRef]
- Gupta, K.J.; Kaiser, W.M. Production and Scavenging of Nitric Oxide by Barley Root Mitochondria. Plant Cell Physiol. 2010, 51, 576–584. [Google Scholar] [CrossRef] [Green Version]
- Corpas, F.J.; Barroso, J.B.; del Río, L.A. Peroxisomes as a source of reactive oxygen species and nitric oxide signal molecules in plant cells. Trends Plant Sci. 2001, 6, 145–150. [Google Scholar] [CrossRef]
- Baudouin, E. The language of nitric oxide signalling. Plant Biol. 2010, 13, 233–242. [Google Scholar] [CrossRef]
- Sanz, L.; Albertos, P.; Mateos, I.; Sánchez-Vicente, I.; Lechón, T.; Fernández-Marcos, M.; Lorenzo, O. Nitric oxide (NO) and phytohormones crosstalk during early plant development. J. Exp. Bot. 2015, 66, 2857–2868. [Google Scholar] [CrossRef]
- Gouvêa, C.; Souza, J.; Magalhães, A.; Martins, I. NO—Releasing substances that induce growth elongation in maize root segments. Plant Growth Regul. 1997, 21, 183–187. [Google Scholar] [CrossRef]
- Pagnussat, G.C.; Simontacchi, M.; Puntarulo, S.; LaMattina, L. Nitric Oxide Is Required for Root Organogenesis. Plant Physiol. 2002, 129, 954–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correa-Aragunde, N.; Graziano, M.; Elamattina, L. Nitric oxide plays a central role in determining lateral root development in tomato. Planta 2004, 218, 900–905. [Google Scholar] [CrossRef]
- Bai, S.; Li, M.; Yao, T.; Wang, H.; Zhang, Y.; Xiao, L.; Wang, J.-Z.; Zhang, Z.; Hu, Y.; Liu, W.; et al. Nitric oxide restrain root growth by DNA damage induced cell cycle arrest in Arabidopsis thaliana. Nitric Oxide 2012, 26, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Lira-Ruan, V.; Napsucialy, S.N.; Dubrovsky, J.G. Heuristic aspect of the lateral root initiation index: A case study of the role of nitric oxide in root branching1. Appl. Plant Sci. 2013, 1, 1300029. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, M.C.; Graziano, M.; Polacco, J.C.; Elamattina, L. Nitric Oxide Functions as a Positive Regulator of Root Hair Development. Plant Signal. Behav. 2006, 1, 28–33. [Google Scholar] [CrossRef] [Green Version]
- Lehner, C.; Kerschbaum, H.H.; Lütz-Meindl, U. Nitric oxide suppresses growth and development in the unicellular green alga Micrasterias denticulata. J. Plant Physiol. 2009, 166, 117–127. [Google Scholar] [CrossRef]
- Estevez, M.S.; Puntarulo, S. Nitric oxide generation upon growth of Antarctic Chlorella sp. cells. Physiol. Plant. 2005, 125, 192–201. [Google Scholar] [CrossRef]
- Pokora, W.; Aksmann, A.; Baścik-Remisiewicz, A.; Dettlaff-Pokora, A.; Rykaczewski, M.; Gappa, M.; Tukaj, Z. Changes in nitric oxide/hydrogen peroxide content and cell cycle progression: Study with synchronized cultures of green alga Chlamydomonas reinhardtii. J. Plant Physiol. 2017, 208, 84–93. [Google Scholar] [CrossRef]
- Thelander, M.; Landberg, K.; Sundberg, E. Auxin-mediated developmental control in the moss Physcomitrella patens. J. Exp. Bot. 2017, 69, 277–290. [Google Scholar] [CrossRef]
- Kofuji, R.; Hasebe, M. Eight types of stem cells in the life cycle of the moss Physcomitrella patens. Curr. Opin. Plant Biol. 2014, 17, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Ponce De León, I.; Schmelz, E.A.; Gaggero, C.; Castro, A.; Álvarez, A.; Montesano, M. Physcomitrella patens activates reinforcement of the cell wall, programmed cell death and accumulation of evolutionary conserved defence signals, such as salicylic acid and 12-oxo-phytodienoic acid, but not jasmonic acid, upon Botrytis cinerea infection. Mol. Plant Pathol. 2012, 13, 960–974. [Google Scholar] [CrossRef]
- Rensing, S.A.; Goffinet, B.; Meyberg, R.; Wu, S.-Z.; Bezanilla, M. The Moss Physcomitrium (Physcomitrella) patens: A Model Organism for Non-Seed Plants. Plant Cell 2020, 32, 1361–1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medina-Andrés, R.; Solano-Peralta, A.; Saucedo-Vázquez, J.P.; Napsucialy-Mendivil, S.; Pimentel-Cabrera, J.A.; Sosa-Torres, M.E.; Dubrovsky, J.G.; Lira-Ruan, V. The Nitric Oxide Production in the Moss Physcomitrella patens is Mediated by Nitrate Reductase. PLoS ONE 2015, 10, e0119400. [Google Scholar] [CrossRef] [PubMed]
- Ashton, N.W.; Cove, D.J. The isolation and preliminary characterisation of auxotrophic and analogue resistant mutants of the moss, Physcomitrella patens. Mol. Genet. Genom. 1977, 154, 87–95. [Google Scholar] [CrossRef]
- Grimsley, N.H.; Ashton, N.W.; Cove, D.J. The production of somatic hybrids by protoplast fusion in the moss, Physcomitrella patens. Mol. Genet. Genom. 1977, 154, 97–100. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Chem. Biol. 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Chiariello, N.R.; Mooney, H.A.; Williams, K. Growth, carbon allocation and cost of plant tissues. In Plant Physiological Ecology: Field Methods and Instrumentation; Pearcy, R.W., Ehleringer, J.R., Mooney, H.A., Rundel, P.W., Eds.; Springer: Dordrecht, The Netherlands, 1989; pp. 327–365. [Google Scholar]
- Burgess, J.; Linstead, P.J. Studies on the growth and development of protoplasts of the moss, Physcomitrella patens, and its control by light. Planta 1981, 151, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, G.I.; Cove, D.J. Light requirements for regeneration of protoplasts o themoss Physcomitrella patens. Planta 1983, 157, 39–45. [Google Scholar] [CrossRef]
- Cove, D.J.; Quatrano, R.S. The Use of Mosses for the Study of Cell Polarity. In New Frontiers in Bryology: Physiology, Molecular Biology and Functional Genomics; Wood, A.J., Oliver, M.J., Cove, D.J., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 189–203. [Google Scholar]
- Thelander, M.; Olsson, T.; Ronne, H. Effect of the energy supply on filamentous growth and development in Physcomitrella patens. J. Exp. Bot. 2005, 56, 653–662. [Google Scholar] [CrossRef] [Green Version]
- Bhatla, S.C.; Kiessling, J.; Reski, R. Observation of polarity induction by cytochemical localization of phenylalkylamine-binding sites in regenerating protoplasts of the moss Physcomitrella patens. Protoplasma 2002, 219, 99–105. [Google Scholar] [CrossRef]
- Ötvös, K.; Pasternak, T.P.; Miskolczi, P.; Domoki, M.; Dorjgotov, D.; Szcs, A.; Bottka, S.; Dudits, D.; Fehér, A. Nitric oxide is required for, and promotes auxin-mediated activation of, cell division and embryogenic cell formation but does not influence cell cycle progression in alfalfa cell cultures. Plant J. 2005, 43, 849–860. [Google Scholar] [CrossRef]
- Prado, A.M.; Porterfield, D.M.; Feijó, J.A. Nitric oxide is involved in growth regulation and re-orientation of pollen tubes. Development 2004, 131, 2707–2714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prado, A.M.; Colaço, R.; Moreno, N.; Silva, A.C.; Feijó, J.A. Targeting of Pollen Tubes to Ovules Is Dependent on Nitric Oxide (NO) Signaling. Mol. Plant 2008, 1, 703–714. [Google Scholar] [CrossRef] [PubMed]
- He, J.-M.; Bai, X.-L.; Wang, R.-B.; Cao, B.; She, X.-P. The involvement of nitric oxide in ultraviolet-B-inhibited pollen germination and tube growth of Paulownia tomentosa in vitro. Physiol. Plant. 2007, 131, 273–282. [Google Scholar] [CrossRef]
- Šírová, J.; Sedlářová, M.; Piterková, J.; Luhová, L.; Petrivalsky, M. The role of nitric oxide in the germination of plant seeds and pollen. Plant Sci. 2011, 181, 560–572. [Google Scholar] [CrossRef]
- Jiménez-Quesada, M.J.; Carmona, R.; Lima-Cabello, E.; Traverso, J.Á.; Castro, A.J.; Claros, M.G.; Alché, J.D.D. Generation of nitric oxide by olive (Olea europaea L.) pollen during in vitro germination and assessment of the S-nitroso- and nitro-proteomes by computational predictive methods. Nitric Oxide 2017, 68, 23–37. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chen, T.; Zhang, C.; Hao, H.; Liu, P.; Zheng, M.; Baluška, F.; Šamaj, J.; Lin, J. Nitric oxide modulates the influx of extracellular Ca2+ and actin filament organization during cell wall construction in Pinus bungeanapollen tubes. New Phytol. 2009, 182, 851–862. [Google Scholar] [CrossRef]
- Benko, P.; Jee, S.; Kaszler, N.; Fehér, A.; Gémes, K. Polyamines treatment during pollen germination and pollen tube elongation in tobacco modulate reactive oxygen species and nitric oxide homeostasis. J. Plant Physiol. 2020, 244, 153085. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cervantes-Pérez, D.; Ortega-García, A.; Medina-Andrés, R.; Batista-García, R.A.; Lira-Ruan, V. Exogenous Nitric Oxide Delays Plant Regeneration from Protoplast and Protonema Development in Physcomitrella patens. Plants 2020, 9, 1380. https://doi.org/10.3390/plants9101380
Cervantes-Pérez D, Ortega-García A, Medina-Andrés R, Batista-García RA, Lira-Ruan V. Exogenous Nitric Oxide Delays Plant Regeneration from Protoplast and Protonema Development in Physcomitrella patens. Plants. 2020; 9(10):1380. https://doi.org/10.3390/plants9101380
Chicago/Turabian StyleCervantes-Pérez, Daniela, Angélica Ortega-García, Rigoberto Medina-Andrés, Ramón Alberto Batista-García, and Verónica Lira-Ruan. 2020. "Exogenous Nitric Oxide Delays Plant Regeneration from Protoplast and Protonema Development in Physcomitrella patens" Plants 9, no. 10: 1380. https://doi.org/10.3390/plants9101380