A Review of Polysaccharide-Zinc Oxide Nanocomposites as Safe Coating for Fruits Preservation
Abstract
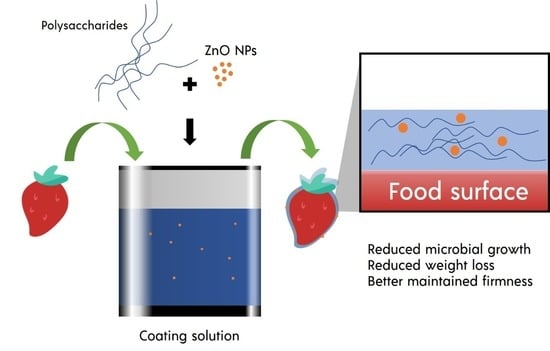
:1. Introduction
2. Basic Concepts of Polysaccharide-Based Safe Coating
3. Effects of Zinc Oxide Nanoparticles on Properties of Polysaccharide-Based Coating
3.1. Chitosan
3.2. Alginate
3.3. Carrageenan
3.4. Carboxymethyl Cellulose
3.5. Pectin
4. Safety Issue of Zinc Oxide Nanoparticles as a Safe Coating Material
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Marsh, K.; Bugusu, B. Food packaging—Roles, materials, and environmental issues: Scientific status summary. J. Food Sci. 2007, 72, R39–R55. [Google Scholar] [CrossRef] [PubMed]
- Arfat, Y.A.; Ejaz, M.; Jacob, H.; Ahmed, J. Deciphering the potential of guar gum/Ag-Cu nanocomposite films as an active food packaging material. Carbohydr. Polym. 2017, 157, 65–71. [Google Scholar] [CrossRef] [PubMed]
- De Silva, R.T.; Mantilaka, M.M.M.G.P.G.; Ratnayake, S.P.; Amaratunga, G.A.J.; Nalin de Silva, K.M. Nano-MgO reinforced chitosan nanocomposites for high performance packaging applications with improved mechanical, thermal and barrier properties. Carbohydr. Polym. 2017, 157, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Liu, Y.; Song, Y.; Han, J.; Pan, H. Rosin modified cellulose nanofiber as a reinforcing and co-antimicrobial agents in polylactic acid /chitosan composite film for food packaging. Carbohydr. Polym. 2018, 183, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Guilbert, S.; Gontard, N.; Cuq, B. Technology and applications of edible protective films. Packag. Technol. Sci. 1995, 8, 339–346. [Google Scholar] [CrossRef]
- Ncama, K.; Magwaza, L.S.; Mditshwa, A.; Tesfay, S.Z. Plant-based edible coatings for managing postharvest quality of fresh horticultural produce: A review. Food Packag. Shelf Life 2018, 16, 157–167. [Google Scholar] [CrossRef]
- Murmu, S.B.; Mishra, H.N. The effect of edible coating based on arabic gum, sodium caseinate and essential oil of cinnamon and lemon grass on guava. Food Chem. 2018, 245, 820–828. [Google Scholar] [CrossRef]
- Joshy, K.S.; Jose, J.; Li, T.; Thomas, M.; Shankregowda, A.M.; Sreekumaran, S.; Kalarikkal, N.; Thomas, S. Application of novel zinc oxide reinforced xanthan gum hybrid system for edible coatings. Int. J. Biol. Macromol. 2020, 151, 806–813. [Google Scholar] [CrossRef]
- Gamage, T.V.; Rehman, M.S. Post harvest handling of foods of plant origin. In Handbook of Food Preservation; Rehman, M.S., Ed.; Marcel Dekker: New York, NY, USA, 1999; pp. 11–46. [Google Scholar]
- Arroyo, B.J.; Bezerra, A.C.; Oliveira, L.L.; Arroyo, S.J.; de Melo, E.A.; Santos, A.M.P. Antimicrobial active edible coating of alginate and chitosan add ZnO nanoparticles applied in guavas (Psidium guajava L.). Food Chem. 2020, 309, 125566. [Google Scholar] [CrossRef]
- Maftoonazad, N.; Ramaswamy, H.S.; Marcotte, M. Evaluation of factors affecting barrier, mechanical and optical properties of pectin-based films using response surface methodology. J. Food Process Eng. 2007, 30, 539–563. [Google Scholar] [CrossRef]
- Moalemiyan, M.; Ramaswamy, H.S.; Maftoonazad, N. Pectin-based edible coating for shelf-life extension of Ataulfo mango. J. Food Process Eng. 2012, 35, 572–600. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Sahraee, S.; Milani, J.M.; Regenstein, J.M.; Kafil, H.S. Protection of foods against oxidative deterioration using edible films and coatings: A review. Food Biosci. 2019, 32, 100451. [Google Scholar] [CrossRef]
- Meindrawan, B.; Suyatma, N.E.; Wardana, A.A.; Pamela, V.Y. Nanocomposite coating based on carrageenan and ZnO nanoparticles to maintain the storage quality of mango. Food Packag. Shelf Life 2018, 18, 140–146. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Tye, Y.Y.; Saurabh, C.K.; Leh, C.P.; Lai, T.K.; Chong, E.W.N.; Nurul Fazita, M.R.; Hafiidz, J.M.; Banerjee, A.; Syakir, M.I. Biodegradable polymer films from seaweed polysaccharides: A review on cellulose as a reinforcement material. Express Polym. Lett. 2017, 11, 244–265. [Google Scholar] [CrossRef]
- Romanazzi, G.; Feliziani, E.; Baños, S.B.; Sivakumar, D. Shelf life extension of fresh fruit and vegetables by chitosan treatment. Crit. Rev. Food Sci. Nutr. 2017, 57, 579–601. [Google Scholar] [CrossRef]
- Cakmak, I.; Kutman, U.B. Agronomic biofortification of cereals with zinc: A review Roles of Zn in human physiology and health. Eur. J. Soil Sci. 2018, 69, 172–180. [Google Scholar] [CrossRef] [Green Version]
- Mwangi, M.N.; Oonincx, D.G.A.B.; Stouten, T.; Veenenbos, M.; Melse-Boonstra, A.; Dicke, M.; van Loon, J.J.A. Insects as sources of iron and zinc in human nutrition. Nutr. Res. Rev. 2018, 31, 248–255. [Google Scholar] [CrossRef]
- Sun, Q.; Li, J.; Le, T. Zinc Oxide Nanoparticle as a Novel Class of Antifungal Agents: Current Advances and Future Perspectives. J. Agric. Food Chem. 2018, 66, 11209–11220. [Google Scholar] [CrossRef]
- Pavlath, A.E.; Orts, W. Edible films and coatings: Why, what, and how? In Edible Films and Coatings for Food Applications; Huber, K.C., Embuscado, M.E., Eds.; Springer: New York, NY, USA, 2009; pp. 1–23. [Google Scholar]
- Nussinovitch, A. Hydrocolloids for coatings and adhesives. In Handbook of Hydrocolloids; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing Series: Shaston, UK, 2009; pp. 760–806. [Google Scholar]
- Andrade, R.D.; Skurtys, O.; Osorio, F.A. Atomizing spray systems for application of edible coatings. Compr. Rev. Food Sci. Food Saf. 2012, 11, 323–337. [Google Scholar] [CrossRef]
- Sipahi, R.E.; Castell-Perez, M.E.; Moreira, R.G.; Gomes, C.; Castillo, A. Improved multilayered antimicrobial alginate-based edible coating extends the shelf life of fresh-cut watermelon (Citrullus lanatus). LWT Food Sci. Technol. 2013, 51, 9–15. [Google Scholar] [CrossRef]
- Weiss, J.; Takhistov, P.; McClements, D.J. Functional materials in food nanotechnology. J. Food Sci. 2006, 71, R107–R116. [Google Scholar] [CrossRef] [Green Version]
- Poverenov, E.; Danino, S.; Horev, B.; Granit, R.; Vinokur, Y.; Rodov, V. Layer-by-layer electrostatic deposition of edible coating on fresh cut melon model: Anticipated and unexpected effects of alginate–chitosan combination. Food Bioprocess Technol. 2014, 7, 1424–1432. [Google Scholar] [CrossRef]
- Skurtys, O.; Acevedo, C.A.; Pedreschi, F.; Enrione, J.; Osorio, F.; Aguilera, J.M.C. Food Hydrocolloid Edible Films and Coatings; Hollingworth, C.S., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2010. [Google Scholar]
- Duan, J.; Wu, R.; Strik, B.C.; Zhao, Y. Effect of edible coatings on the quality of fresh blueberries (Duke and Elliott) under commercial storage conditions. Postharvest Biol. Technol. 2011, 59, 71–79. [Google Scholar] [CrossRef]
- Youssef, A.M.; Assem, F.M.; El-Sayed, S.M.; Salama, H.; Abd El-Salam, M.H. Utilization of Edible Films and Coatings as Packaging Materials for Preservation of Cheeses. J. Packag. Technol. Res. 2017, 1, 87–99. [Google Scholar] [CrossRef]
- Ahmadian-Yazdi, M.R.; Zabihi, F.; Habibi, M.; Eslamian, M. Effects of Process Parameters on the Characteristics of Mixed-Halide Perovskite Solar Cells Fabricated by One-Step and Two-Step Sequential Coating. Nanoscale Res. Lett. 2016, 11, 408. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Blawert, C.; Lamaka, S.V.; Snihirova, D.; Lu, X.; Di, S.; Zheludkevich, M.L. Corrosion protection properties of inhibitor containing hybrid PEO-epoxy coating on magnesium. Corros. Sci. 2018, 140, 99–110. [Google Scholar] [CrossRef]
- Tharanathan, R.N. Biodegradable films and composite coatings: Past, present and future. Trends Food Sci. Biotechnol. 2003, 14, 71–78. [Google Scholar] [CrossRef]
- Norton, T.; Sun, D.W. Computational fluid dynamics (cfd)—An effective and efficient design and analysis tool for the food industry: A review. Trends Food Sci. Biotechnol. 2006, 17, 600–620. [Google Scholar] [CrossRef]
- Guillemin, A.; Degraeve, P.; Noel, C.; Saurel, R. Influence of impregnation solution viscosity and osmolarity on solute uptake during vacuum impregnation of apple cubes (var. Granny smith). J. Food Eng. 2008, 86, 475–483. [Google Scholar] [CrossRef]
- Vargas, M.; Chiralt, A.; Albors, A.; Gonzalez-Martinez, C. Effect of chitosan-based edible coatings applied by vacuum impregnation on quality preservation of fresh-cut carrot. Postharvest Biol. Technol. 2009, 51, 263–271. [Google Scholar] [CrossRef]
- Parreidt, T.S.; Schmid, M.; Muller, K. Effect of dipping and vacuum impregnation coating techniques with alginate based coating on physical quality parameters of cantaloupe melon. J. Food Sci. 2018, 83, 929–936. [Google Scholar] [CrossRef]
- Trandafilović, L.V.; Božanić, D.K.; Dimitrijević-Branković, S.; Luyt, A.S.; Djoković, V. Fabrication and antibacterial properties of ZnO-alginate nanocomposites. Carbohydr. Polym. 2012, 88, 263–269. [Google Scholar] [CrossRef]
- Yousuf, B.; Qadri, O.S.; Srivastava, A.K. Recent developments in shelf-life extension of fresh-cut fruits and vegetables by application of different edible coatings: A review. LWT Food Sci. Technol. 2018, 89, 198–209. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.W. Properties and characterization of bionanocomposite films prepared with various biopolymers and ZnO nanoparticles. Carbohydr. Polym. 2014, 106, 190–199. [Google Scholar] [CrossRef]
- Mohammadi, H.; Kamkar, A.; Misaghi, A. Nanocomposite films based on CMC, okra mucilage and ZnO nanoparticles: Physico mechanical and antibacterial properties. Carbohydr. Polym. 2018, 181, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Oun, A.A.; Rhim, J.-W. Preparation of multifunctional chitin nanowhiskers/ZnO-Ag NPs and their effect on the properties of carboxymethyl cellulose-based nanocomposite film. Carbohydr. Polym. 2017, 169, 467–479. [Google Scholar] [CrossRef]
- Lavinia, M.; Hibarturrahman, S.N.; Harinata, H.; Wardana, A.A. Antimicrobial activity and application of nanocomposite coating from chitosan and ZnO nanoparticle to inhibit microbial growth on fresh-cut papaya. Food Res. 2019, 4, 307–311. [Google Scholar] [CrossRef]
- Emamifar, A.; Bavaisi, S. Nanocomposite coating based on sodium alginate and nano-ZnO for extending the storage life of fresh strawberries (Fragaria × ananassa Duch.). J. Food Meas. Charact. 2020, 14, 1012–1024. [Google Scholar] [CrossRef]
- Koushesh Saba, M.; Amini, R. Nano-ZnO/carboxymethyl cellulose-based active coating impact on ready-to-use pomegranate during cold storage. Food Chem. 2017, 232, 721–726. [Google Scholar] [CrossRef]
- Romadhan, M.F.; Pujilestari, S. Sintesis nanopartikel ZnO dan aplikasinya sebagai edible coating berbasis pektin untuk memperpanjang umur simpan buah belimbing. Jurnal Agroindustri Halal 2019, 5, 030–038. [Google Scholar] [CrossRef]
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.V.; Tamura, H. Biomedical applications of chitin and chitosan based nanomaterials—A short review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S.-K. Chitosan composites for bone tissue engineering—An overview. Mar. Drugs 2010, 8, 2252–2266. [Google Scholar] [CrossRef] [Green Version]
- Croisier, F.; Jerome, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef] [Green Version]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Campos, C.A.; Gerschenson, L.N.; Flores, S.K. Development of edible films and coatings with antimicrobial activity. Food Bioprocess Technol. 2010, 4, 849–875. [Google Scholar] [CrossRef]
- Ferreira, C.O.; Nunes, C.A.; Delgadillo, I.; Lopes-da-Silva, J.A. Characterization of chitosan-whey protein films at acid pH. Food Res. Int. 2009, 42, 807–813. [Google Scholar] [CrossRef]
- Khor, E.; Lim, L.Y. Implantable applications of chitin and chitosan. Biomaterials 2003, 24, 2339–2349. [Google Scholar] [CrossRef]
- Li, Q.; Dunn, E.T.; Grandmaison, E.W.; Goosen, M.F.A. Applications and properties of chitosan. J. Bioact. Compat. Polym. 1992, 7, 370–397. [Google Scholar] [CrossRef]
- Romanazzi, G.; Feliziani, E.; Santini, M.; Landi, L. Effectiveness of postharvest treatment with chitosan and other resistance inducers in the control of storage decay of strawberry. Postharvest Biol. Technol. 2013, 75, 24–27. [Google Scholar] [CrossRef]
- Anugrah, D.S.B.; Ramesh, K.; Kim, M.; Hyun, K.; Lim, K.T. Near-infrared light-responsive alginate hydrogels based on diselenide-containing cross-linkage for on demand degradation and drug release. Carbohydr. Polym. 2019, 223, 115070. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parreidt, T.S.; Müller, K.; Schmid, M. Alginate-based edible films and coatings for food packaging applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shojaee-Aliabadi, S.; Hosseini, H.; Mohammadifar, M.A.; Mohammadi, A.; Ghasemlou, M.; Hosseini, S.M.; Khaksar, R. Characterization of κ-carrageenan films incorporated plant essential oils with improved antimicrobial activity. Carbohydr. Polym. 2014, 101, 582–591. [Google Scholar] [CrossRef]
- Pettignano, A.; Charlot, A.; Fleury, E. Carboxyl-functionalized derivatives of carboxymethyl cellulose: Towards advanced biomedical applications. Polym. Rev. 2019, 59, 510–560. [Google Scholar] [CrossRef]
- Nešić, A.; Gordić, M.; Davidović, S.; Radovanović, Ž.; Nedeljković, J.; Smirnova, I.; Gurikov, P. Pectin-based nanocomposite aerogels for potential insulated food packaging application. Carbohydr. Polym. 2018, 195, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Bumbudsanpharoke, N.; Choi, J.; Park, H.J.; Ko, S. Zinc migration and its effect on the functionality of a low density polyethylene-ZnO nanocomposite film. Food Packag. Shelf Life 2019, 20. [Google Scholar] [CrossRef]
- Liu, J.; Hu, J.; Liu, M.; Cao, G.; Gao, J.; Luo, Y. Migration and characterization of nano-zinc oxide from polypropylene food containers. Am. J. Food Technol. 2016, 11, 159–164. [Google Scholar] [CrossRef] [Green Version]
- Jin, T.; Sun, D.; Su, J.Y.; Zhang, H.; Sue, H.-J. Antimicrobial efficacy of zinc oxide quantum dots against Listeria monocytogenes, Salmonella enteritidis, and Escherichia coli O157:H7. J. Food Sci. 2009, 74, 46–52. [Google Scholar] [CrossRef]
- Barkhordari, A.; Hekmatimoghaddam, S.; Jebali, A.; Khalili, M.A.; Talebi, A.; Noorani, M. Effect of zinc oxide nanoparticles on viability of human spermatozoa. Int. J. Reprod. Biomed. 2013, 11, 767–771. [Google Scholar]
- Wahab, R.; Kaushik, N.K.; Kaushik, N.; Choi, E.H.; Umar, A.; Dwivedi, S.; Musarrat, J.; Al-Khedhairy, A.A. ZnO Nanoparticles Induces Cell Death in Malignant Human T98G Gliomas, KB and Non-Malignant HEK Cells. J. Biomed. Nanotechnol. 2013, 9, 1181–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namvar, F.; Rahman, H.S.; Mohamad, R.; Azizi, S.; Tahir, P.M.; Chartrand, M.S.; Yeap, S.K. Cytotoxic effects of biosynthesized zinc oxide nanoparticles on murine cell lines. Evid. Based Complement. Altern. Med. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]




| Polysaccharide | Additives | Coating Method | Fruit | Storage Condition | Effect of Coating | References |
|---|---|---|---|---|---|---|
| Chitosan 5% w/v | ZnO 1% v/v gel | Dipping | Guava | 20 days at 21 ± 1 °C and 80% RH | Reduced weight loss, color, and firmness are better maintained; no external injuries until end of storage; and reduced ripening index ratio (SS/TA) | [10] |
| Chitosan (3 g in 0.4 L coating solution) | ZnO 0.005%, 0.006%, 0.010%, 0.020%, and 0.027% w/w coating solution (611.30 nm) | Dipping | Fresh-cut papaya | 12 days, 10 °C | Reduced microbial growth | [42] |
| Alginate 1.5% w/v | ZnO 0.25, 0.75, and 1.25 g/L (30–50 nm) | Dipping | Strawberry | 20 days, 1 °C, RH 95% | Reduced microbial growth, reduced weight loss, better maintained firmness, lower increases in soluble solid, lower decreases in acidity, lower decreases in anthocyanin, phenolic, and antioxidant activities, lower increases in peroxidase activity, and lower decreases in superoxide dismutase activity | [43] |
| Alginate 5% w/v | ZnO 1% w/v gel | Dipping | Guava | 20 days at 21 ± 1 °C and RH 80 | None | [10] |
| Alginate–chitosan (90%–10%) 5% w/v | ZnO 1% w/v gel | Dipping | Guava | 20 days at 21 ± 1 °C and RH 80 | Firmness are better maintained and prevent external injuries | [10] |
| Carrageenan 0.8 g in 0.1 L solution | ZnO 0.5% and 1% w/w of carrageenan | Dipping | Mango | 20 °C and RH 61% | Reduced weight loss, reduced CO2 production, better maintained total acidity, better maintained color, and better maintained textural appearance | [15] |
| CMC 0.5% w/v | ZnO 0.1% and 0.2% w/v (30–100 nm) | Dipping | Pomegranate arils | 12 days, 4 °C and RH 90% | Reduced weight loss, reduced vitamin C loss, reduced anthocyanin and phenolic content loss, and higher antioxidant activities | [44] |
| Pectin 10 g in 1 L solution | ZnO 0.1 g inside 1 L solution | Dipping | Star fruit | 8 days at 25 °C | Reduced weight loss, reduced browning index and redness value, and reduced physical damage | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anugrah, D.S.B.; Alexander, H.; Pramitasari, R.; Hudiyanti, D.; Sagita, C.P. A Review of Polysaccharide-Zinc Oxide Nanocomposites as Safe Coating for Fruits Preservation. Coatings 2020, 10, 988. https://doi.org/10.3390/coatings10100988
Anugrah DSB, Alexander H, Pramitasari R, Hudiyanti D, Sagita CP. A Review of Polysaccharide-Zinc Oxide Nanocomposites as Safe Coating for Fruits Preservation. Coatings. 2020; 10(10):988. https://doi.org/10.3390/coatings10100988
Chicago/Turabian StyleAnugrah, Daru Seto Bagus, Hugo Alexander, Rianita Pramitasari, Dwi Hudiyanti, and Christyowati Primi Sagita. 2020. "A Review of Polysaccharide-Zinc Oxide Nanocomposites as Safe Coating for Fruits Preservation" Coatings 10, no. 10: 988. https://doi.org/10.3390/coatings10100988