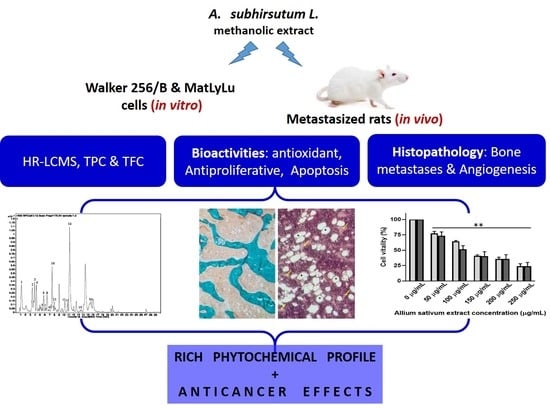
Allium subhirsutum L. as a Potential Source of Antioxidant and Anticancer Bioactive Molecules: HR-LCMS Phytochemical Profiling, In Vitro and In Vivo Pharmacological Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material and Preparation
2.3. Identification of Bioactive Compounds by High Resolution-Liquid Chromatography Mass Spectroscopy (HR-LCMS)
2.4. In Silico ADME and Toxicity Profiles
2.5. Antioxidant Assays
2.5.1. Reducing Power Assay
2.5.2. Antiradical Activity against DPPH
2.6. In Vitro Assays
2.6.1. Malignant MatLyLu and Walker 256/B Cell Lines and Culture Conditions
2.6.2. In Vitro Anticancer Assessment and MTT Assay
2.6.3. Hoechst 33,342 Assay
2.7. Animal Farming and Surgical Procedure
2.8. In Vivo Assays
2.8.1. In Vivo Anticancer Activity of the AS Extract on Breast Cancer Skeletal Metastases
- Control (CTRL) group: composed of six control-operated rats that received an injection of physiological saline in the right femur.
- W256 group: composed of six metastasized rats that received an injection of malignant Walker 256/B cells (104 in 10 µL) in the right femur.
- W256-ASE group: composed of six metastasized rats that received an injection of 5 × 104 malignant Walker 256/B cells in the right femur as above and were treated with ASE. ASE, dissolved in olive oil (4 mg/mL), was administered daily by gavage at a final concentration of 200 mg/kg BW.
- ASE group: composed of six control-operated rats that received an injection of physiological saline in the right femur and were treated with ASE (200 mg/kg BW).
2.8.2. In Vivo Angiogenesis Study
2.9. Statistical Analyses
3. Results and Discussion
3.1. Phytochemical Composition: HR-LCMS and Bioinformatics’ Findings
3.2. Total Polyphenol and Flavonoid Contents and Antioxidant Properties
3.3. In Vitro Findings: Cytotoxicity and Apoptotic Induction
3.4. General In Vivo Findings
3.5. In Vivo Findings: Bone Metastasis and Angiogenesis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the global burden of disease study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Zambrowski, J.J. Costs of cancer care. Rev. Prat. 2016, 66, 489–493. [Google Scholar] [PubMed]
- Bharti, A.C.; Aggarwal, B.B. Nuclear factor-kappa B and cancer: Its role in prevention and therapy. Biochem. Pharmacol. 2002, 101, 1053–1062. [Google Scholar] [CrossRef]
- Badraoui, R.; Rebai, T. Effect of malignant ascites on antioxidative potency of two tumoral cells-induced bone metastases: Walker 256/B and MatLyLu. Exp. Toxicol. Pathol. 2012, 64, 65–68. [Google Scholar] [CrossRef]
- Blouin, S.; Baslé, M.F.; Chappard, D. Rat models of bone metastases. Clin. Exp. Metastasis 2005, 22, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Mao-Ying, Q.-L.; Zhao, J.; Dong, Z.-Q.; Wang, J.; Yu, J.; Yan, M.-F.; Zhang, Y.-Q.; Wu, G.-C.; Wang, Y.-Q. A rat model of bone cancer pain induced by intra-tibia inoculation of Walker 256 mammary gland carcinoma cells. Biochem. Biophys. Res. Commun. 2006, 345, 1292–1298. [Google Scholar] [CrossRef]
- Sadremomtaz, A.; Mansouri, K.; Alemzadeh, G.; Safa, M.; Rastaghi, A.E.; Asghari, S.M. Dual blockade of VEGFR1 and VEGFR2 by a novel peptide abrogates VEGF-driven angiogenesis, tumor growth, and metastasis through PI3K/AKT and MAPK/ERK1/2 pathway. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2688–2700. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, T.; Zhang, S.; Shan, J. Prognostic values of F-box members in breast cancer: An online database analysis and literature review. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [Green Version]
- Badraoui, R.; Blouin, S.; Moreau, M.F.; Gallois, Y.; Rebaï, T.; Sahnoun, Z.; Baslé, M.; Chappard, D. Effect of alpha tocopherol acetate in Walker 256/B cells-induced oxidative damage in a rat model of breast cancer skeletal metastases. Chem. Interact. 2009, 182, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Badraoui, R.; Boubakri, M.; Bedbabiss, M.; Ben-Nasr, H.; Rebai, T. Walker 256/B malignant breast cancer cells enhance femur angiogenesis and disrupt hematological parameters in rats. Tumor Biol. 2014, 35, 3663–3670. [Google Scholar] [CrossRef]
- Nyangoga, H.; Aguedo, E.; Goyenvalle, E.; Baslé, M.F.; Chappard, D. Three-dimensional characterization of the vascular bed in bone metastasis of the rat by microcomputed tomography (MicroCT). PLoS ONE 2011, 6, e17336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blouin, S.; Moreau, M.F.; Baslé, M.F.; Chappard, D. Relations between radiograph texture analysis and microcomputed tomography in two rat models of bone metastases. Cells Tissues Organs 2006, 182, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Badraoui, R.; Ben-Nasr, H.; Amamou, S.; El-May, M.V.; Rebai, T. Walker 256/B malignant breast cancer cells disrupt osteoclast cytomorphometry and activity in rats: Modulation by α–tocophrerol acetate. Pathol. Res. Pract. 2014, 210, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Guesmi, F.; Saidi, I.; Soussi, R.; Hfaiedh, N.; Landoulsi, A. Antioxidant potential of four species of natural product and therapeutic strategies for cancer through suppression of viability in the human multiple myeloma cell line U266. Biomed. Environ. Sci. 2019, 32, 22–33. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Yuan, W.; Li, S.; Gupta, S.C. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: Identification of novel components of turmeric. Mol. Nutr. Food Res. 2013, 57, 1529–1542. [Google Scholar] [CrossRef]
- Emir, A.; Emir, C.; Yıldırım, H. Characterization of phenolic profile by LC-ESI-MS/MS and enzyme inhibitory activities of two wild edible garlic: Allium nigrum L. and Allium subhirsutum L. J. Food Biochem. 2020, 44, e13165. [Google Scholar] [CrossRef]
- Štajner, D.; Popovic, B.M.; Čanadanović-Brunet, J.; Igić, R.S. Antioxidant and free radical scavenging of Allium roseum and Allium subhirsutum. Phytother. Res. 2008, 22, 1469–1471. [Google Scholar] [CrossRef]
- Rivlin, R.S. Historical perspective on the use of garlic. J. Nutr. 2001, 131, 951S–954S. [Google Scholar] [CrossRef] [Green Version]
- Craig, W.J. Health-promoting properties of common herbs. Am. J. Clin. Nutr. 1999, 70, 491s–499s. [Google Scholar] [CrossRef]
- Powolny, A.A.; Singh, S.V. Multitargeted prevention and therapy of cancer by diallyl trisulfide and related Allium vegetables-derived organosulfur compounds. Cancer Lett. 2008, 269, 305–314. [Google Scholar] [CrossRef] [Green Version]
- Arunkumar, A.; Vijayababu, M.R.; Srinivasan, N.; Aruldhas, M.M.; Arunakaran, J. Garlic compound, dially disulfide induces cell cycle arrest in prostate cancer cell line PC-3. Mol. Cell. Biochem. 2006, 288, 107–113. [Google Scholar] [CrossRef]
- Bianchini, F.; Vainio, H. Allium vegetables and organosulfur compounds: Do they help prevent cancer? Environ. Health Perspect. 2001, 109, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.-Y.; Wallar, G.; Zhou, J.-Y.; Yang, J.; Han, R.-Q.; Wang, P.-H.; Liu, A.; Gu, X.-P.; Zhang, X.-F.; Wang, X.-S.; et al. Consumption of garlic and its interactions with tobacco smoking and alcohol drinking on esophageal cancer in a Chinese population. Eur. J. Cancer Prev. 2019, 28, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Dziri, S.; Hassen, I.; Fatnassi, S.; Mrabet, Y.; Casabianca, H.; Hanchi, B.; Hosni, K. Phenolic constituents, antioxidant and antimicrobial activities of rosy garlic (Allium roseum var. odoratissimum). J. Funct. Foods 2012, 4, 423–432. [Google Scholar] [CrossRef]
- Fang, J.; Liu, C.; Wang, Q.; Lin, P.; Cheng, F. In silico polypharmacology of natural products. Brief. Bioinform. 2018, 19, 1153–1171. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef]
- Brand-William, W.; Cuvellier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Sylvester, P.W. Optimization of the tetrazolium dye (MTT) colorimetric assay for cellular growth and viability. Methods Mol. Biol. 2011, 716, 157–168. [Google Scholar] [CrossRef]
- Badraoui, R.; Amri, N.; Zammel, N.; Chaabane, R.; Rebai, T. Corticosteroid treatment exacerbates bone osteopenia in mice with gonadal hormone deficiency–induced osteoporosis. Eur. J. Pharmaceut. Sci. 2017, 105, 41–46. [Google Scholar] [CrossRef]
- Sut, S.; Maggi, F.; Bruno, S.; Badalamenti, N.; Quassinti, L.; Bramucci, M.; Beghelli, D.; Lupidi, G.; Dall’Acqua, S. Hairy garlic (Allium subhirsutum) from Sicily (Italy): LC-DAD-MSn analysis of secondary metabolites and in vitro biological properties. Molecules 2020, 25, 2837. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, A.; Sudisha, J.; El-Sayed, M.; Ito, S.; Ikeda, T.; Yamauchi, N.; Shigyo, M. Aginoside saponin, a potent antifungal compound, and secondary metabolite analyses from Allium nigrum L. Phytochem. Lett. 2013, 6, 274–280. [Google Scholar] [CrossRef]
- Chen, X.; Ahn, D.U. Antioxidant activities of six natural phenolics against lipid oxidation induced by Fe2+ or ultraviolet light. J. Am. Oil Chem. Soc. 1998, 47, 1717–1721. [Google Scholar] [CrossRef]
- Ben Sghaier, M.B.; Ismail, M.B.; Bouhlel, I.; Ghedira, K.; Chekir-Ghedira, L. Leaf extracts from Teucrium ramosissimum protect gainst DNA damage in human lymphoblast cell K562 and enhance antioxidant, antigenotoxic and antiproliferative activity. Environ. Toxicol. Pharmacol. 2016, 44, 44–52. [Google Scholar] [CrossRef]
- Arumai Selvan, D.; Mahendiran, D.; Senthil Kumar, R.; Kalilur Rahiman, A. Garlic, green tea and turmeric extracts-mediated green synthesis of silver nanoparticles: Phytochemical, antioxidant and in vitro cytotoxicity studies. J. Photochem. Photobiol. B 2018, 180, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K. Protective effect of garlic on cellular senescence in UVB-Exposed HaCaT human keratinocytes. Nutrients 2016, 8, 464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amoodizaj, F.F.; Baghaeifar, S.; Taheri, E.; Jadid, M.F.S.; Safi, M.; Sani, N.S.; Hajazimian, S.; Isazadeh, A.; Shanehbandi, D. Enhanced anticancer potency of doxorubicin in combination with curcumin in gastric adenocarcinoma. J. Biochem. Mol. Toxicol. 2020, e22486. [Google Scholar] [CrossRef]
- Kim, C.; Kim, B. Anti-cancer natural products and their bioactive compounds inducing ER stress-mediated apoptosis: A review. Nutrients 2018, 10, 1021. [Google Scholar] [CrossRef] [Green Version]
- Dutta, S.; Mahalanobish, S.; Saha, S.; Ghosh, S.; Sil, P.C. Natural products: An upcoming therapeutic approach to cancer. Food Chem. Toxicol. 2019, 128, 240–255. [Google Scholar] [CrossRef]
- Millimouno, F.M.; Dong, J.; Yang, L.; Li, J.; Li, X. Targeting apoptosis pathways in cancer and perspectives with natural compounds from mother nature. Cancer Prev. Res. 2014, 7, 1081–1107. [Google Scholar] [CrossRef] [Green Version]
- Lv, Y.; So, K.F.; Wong, N.K.; Xiao, J. Anti-cancer activities of S-allylmercaptocysteine from aged garlic. Chin. J. Nat. Med. 2019, 17, 43–49. [Google Scholar] [CrossRef]
- Das, A.; Banik, N.L.; Ray, S.K. Garlic compounds generate reactive oxygen species leading to activation of stress kinases and cysteine proteases for apoptosis in human T98G and U87MG cells. Cancer 2007, 110, 1083–1095. [Google Scholar] [CrossRef]
- Zhang, B.; Meng, M.; Xiang, S.; Cao, Z.; Xu, X.; Zhao, Z.; Zhang, T.; Chen, B.; Yang, P.; Li, Y.; et al. Selective activation of tumor-suppressive MAPKP signaling pathway by triptonide effectively inhibits pancreatic cancer cell tumorigenicity and tumor growth. Biochem. Pharmacol. 2019, 166, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Niu, X.; Jin, R.; Xu, F.; Ding, J.; Zhang, L.; Huang, Z. Triptonide inhibits metastasis potential of thyroid cancer cells via astrocyte elevated gene-1. Transl. Cancer Res. 2020, 9, 1195–1204. [Google Scholar] [CrossRef]
- Ashrafi, S.; Shapouri, R.; Shirkhani, A.; Mahdavi, M. Anti-tumor effects of propranolol: Adjuvant activity on a transplanted murine breast cancer model. Biomed. Pharmacol. 2018, 104, 45–51. [Google Scholar] [CrossRef]
- Soheilyfar, S.; Velashjerdi, Z.; Hajizadeh, Y.S.; Maroufi, N.F.; Amini, Z.; Khorrami, A.; Azimian, S.H.; Isazadeh, A.; Taefehshokr, S.; Taefehshokr, N. In vivo and in vitro impact of miR-31 amd miR-143 on the suppression of metastasis and invasion in breast cancer. J. Balkan Union Oncol. 2018, 23, 1290–1296. [Google Scholar]
- Shenoy, P.A.; Kuo, A.; Vetter, I.; Smith, M.T.P. The Walker 256 breast cancer cell-induced bone pain model in rats. Front. Pharmacol. 2016, 7, 286. [Google Scholar] [CrossRef] [Green Version]
- Sansone, F.; Mencherini, T.; Picerno, P.; Lauro, M.R.; Cerrato, M.; Aquino, R.P. Development of Health Products from Natural Sources. Curr. Med. Chem. 2019, 26, 4606–4630. [Google Scholar] [CrossRef]
- Hecht, F.; Pessoa, C.F.; Gentile, L.B.; Rosenthal, D.; Carvalho, D.P.; Fortunato, R.S. The role of oxidative stress on breast cancer development and therapy. Tumor Biol. 2016, 37, 4281–4291. [Google Scholar] [CrossRef]
- Mao-Ying, Q.-L.; Wang, X.-W.; Yang, C.-J.; Li, X.; Mi, W.-L.; Wu, G.-C.; Wang, Y.-Q. Robust spinal neuroinflammation mediates mechanical allodynia in Walker 256 induced bone cancer rats. Mol. Brain 2012, 5, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez-Valerio, G.; Casanovas, O. Angiogenesis and metabolism: Entwined for therapy resistance. Trends Cancer 2017, 3, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Bhat, T.A.; Singh, R.P. Tumor angiogenesis—A potential target in cancer chemoprevention. Food Chem. Toxicol. 2008, 46, 1334–1345. [Google Scholar] [CrossRef] [PubMed]
- Kerbel, R.S. Tumor angiogenesis. N. Engl. J. Med. 2008, 358, 2039–2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| N° | Compounds | Class of Compounds | RT | MW | Formula | [m/z]- | [m/z]+ |
|---|---|---|---|---|---|---|---|
| 1 | Methyl N-(amethylbutyryl) glycine | Amino Acid | 1.088 | 173.1041 | C8 H15 N O3 | - | 156.1004 |
| 2 | Bis (2-hydroxypropyl) amine | Amino alcohol | 1.377 | 133.1113 | C6 H15 N O2 | - | 156.0986 |
| 3 | Cepharanthine | Alkaloid | 4.265 | 606.2669 | C37 H38 N2 O6 | 128.0394 | - |
| 4 | 2-methylene-5-(2,5dioxotetrahydrofuran-3-yl)-6-oxo--10,10-dimethylbicyclo [7:2:0] undecane | Amide | 5.394 | 304.1626 | C18 H24 O4 | - | 120.0795 |
| 5 | (22S)-1alpha,22,25-trihydroxy-26,27-dimethyl-23,23,24,24-tetradehydro-24ahomovitamin D3/(22S)-1al | - | 5.647 | 470.3446 | C30 H46 O4 | - | 210.1465 |
| 6 | L-4-Hydroxy-3-methoxy- amethylphenylalanine | Amino Acid | 6.247 | 225.1012 | C11 H15 N O4 | - | 206.0785 |
| 7 | N-(2-fluro-ethyl) arachidonoyl amine | Fatty Amide | 6.366 | 349.2812 | C22 H36 F N O | - | 332.2789 |
| 8 | 1-nonadecanoyl-2- (5Z,8Z,11Z,14Z,17Zeicosapentaenoyl)-sn-glycerol | Glycerolipid | 6.544 | 656.5219 | C42 H72 O5 | - | 210.1477 |
| 9 | TG(16:1(9Z)/17:2(9Z,12Z)/20: 5(5Z,8Z,11Z,14Z,17Z))[iso6] | Glycerolipid | 7.05 | 860.7058 | C56 H92 O6 | - | 210.1471 |
| 10 | L-4-Hydroxy-3-methoxy-amethylphenylalanine | Amino Acid | 7.311 | 225.1012 | C11 H15 N O4 | - | 206.0799 |
| 11 | 11 alpha-acetoxykhivorin | Limonoid | 7.44 | 644.2711 | C34 H44 O12 | - | 177.0531 |
| 12 | Tuberonic acid | Octadecanoid | 7.93 | 226.1192 | C12 H18 O4 | - | 227.1282 |
| 13 | Methyl gamboginate | - | 8.226 | 662.2854 | C39 H47 Cl O7 | - | 323.0934 |
| 14 | Dihydrodeoxystreptomycin | Aminoglycoside antibiotic | 8.968 | 567.2883 | C21 H41 N7 O11 | - | 99.0444 |
| 15 | 6 alpha-Hydroxy Castasterone | Sterol Lipid | 9.349 | 466.3618 | C28 H50 O5 | 447.3427 | - |
| 16 | C16 Sphinganine | Cationic Sphingoid | 10.222 | 273.2653 | C16 H35 N O2 | - | 274.2751 |
| 17 | 3beta,7alpha,12alpha-Trihydroxy-5alpha-cholestan-26-oic acid | Sterol Lipid | 10.811 | 450.3325 | C27 H46 O5 | - | 271.2014 |
| 18 | 4-Oxomytiloxanthin | Isoprenoid | 10.814 | 612.3855 | C40 H52 O5 | - | 253.1909 |
| 19 | Sebacic acid | Fatty Acyl | 11.057 | 202.1211 | C10 H18 O4 | - | 227.1261 |
| 20 | Tuberonic acid | Octadecanoid | 11.101 | 226.1203 | C12 H18 O4 | - | 227.1230 |
| 21 | 6-Deoxo castasterone | Sterol Lipid | 11.65 | 450.366 | C28 H50 O4 | 431.3475 | - |
| 22 | Linolenoyl lysolecithin | - | 13.48 | 517.3154 | C26 H48 N O7 P | - | 184.0723 |
| 23 | 19-Amino-16-hydroxy-16-oxido-10-oxo-11,15,17-trioxa-165-phosphanonadecan-13-ylundecanoate GPETn(10:0/11:0)[U] | Fatty Acyls | 14.714 | 537.3419 | C26 H52 N O8 P | - | 184.0718 |
| 24 | 3beta,7alpha,12alpha-Trihydroxy-5alpha-cholestan- 26-oic acid | Sterol Lipid | 15.276 | 450.3329 | C27 H46 O5 | - | 271.2038 |
| 25 | N-(2-hydroxyethyl)stearamide | Lipid | 19.734 | 327.3122 | C20 H41 N O2 | - | 57.0693 |
| 26 | 2,2-difluoro-hexadecanoic acid | Fatty Acid | 26.968 | 292.2227 | C16 H30 F2 O2 | 309.1819 | - |
| N° | Compounds | Class of Compounds | RT | MW | Formula | [m/z]- | [m/z]+ |
|---|---|---|---|---|---|---|---|
| 27 | Tyr Trp Phe | Small Peptide | 0.979 | 514.2104 | C29 H30 N4 O5 | 50.0155 | - |
| 28 | Asn Asn Asn | Small Peptide | 1.053 | 360.1374 | C12 H20 N6 O7 | 89.0278 | - |
| 29 | Pro Leu | Small Peptide | 1.379 | 228.1455 | C11 H20 N2 O3 | - | 229.1516 |
| 30 | Cys Tyr Trp | Small Peptide | 3.335 | 470.1557 | C23 H26 N4 O5 S | - | 145.0292 |
| 31 | His Asp | Small Peptide | 3.648 | 270.0931 | C10 H14 N4 O5 | 56.0320 | - |
| 32 | Glu Thr | Small Peptide | 3.729 | 248.1015 | C9 H16 N2 O6 | - | 229.0795 |
| 33 | Thr Asp Asn | Small Peptide | 4.031 | 348.134 | C12 H20 N4 O8 | - | 170.0204 |
| 34 | Cys Tyr Trp | Small Peptide | 4.054 | 470.1559 | C23 H26 N4 O5 S | - | 120.0791 |
| 35 | Phe Glu | Small Peptide | 4.211 | 294.1201 | C14 H18 N2 O5 | - | 120.0805 |
| 36 | Asp Arg Tyr | Small Peptide | 4.264 | 452.2018 | C19 H28 N6 O7 | - | 278.0983 |
| 37 | Phe Pro | Small Peptide | 4.518 | 262.1305 | C14 H18 N2 O3 | - | 146.0578 |
| 38 | Val Ser Cys | Small Peptide | 6.772 | 307.1227 | C11 H21 N3 O5 S | - | 128.0688 |
| 39 | Asn Gln Ala | Small Peptide | 7.526 | 331.1507 | C12 H21 N5 O6 | 148.0574 | - |
| 40 | Val Glu Asp | Small Peptide | 7.59 | 361.151 | C14 H23 N3 O8 | - | 177.0529 |
| 41 | Gly Tyr Lys | Small Peptide | 7.995 | 366.19 | C17 H26 N4 O5 | - | 227.1255 |
| 42 | Lys Arg Lys | Small Peptide | 15.311 | 428.3053 | C18 H38 N8 O4 | - | 271.2035 |
| Entry | 5 | 3 | 10 | 12 | 19 | 20 | 21 | 22 | 24 | 25 | 27 | 33 | 35 | 36 | 38 | 42 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Physicochemical Properties/Lipophilicity/Drug-likeness | ||||||||||||||||
| Molecular Weight | 228.29 | 270.24 | 294.3 | 262.3 | 466.69 | 450.69 | 292.41 | 173.21 | 133.19 | 304.38 | 225.24 | 226.27 | 273.45 | 450.65 | 202.25 | 327.55 |
| Num. heavy atoms | 16 | 19 | 21 | 19 | 33 | 32 | 20 | 12 | 9 | 22 | 16 | 16 | 19 | 32 | 14 | 23 |
| Num. arom. heavy atoms | 0 | 5 | 6 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 0 |
| Fraction Csp3 | 0.82 | 0.4 | 0.36 | 0.43 | 1 | 1 | 0.94 | 0.75 | 1 | 0.72 | 0.36 | 0.67 | 1 | 0.96 | 0.8 | 0.95 |
| Num. rotatable bonds | 6 | 8 | 9 | 5 | 5 | 5 | 14 | 6 | 4 | 1 | 4 | 6 | 14 | 6 | 9 | 19 |
| Num. H-bond acceptors | 4 | 7 | 6 | 4 | 5 | 4 | 4 | 3 | 3 | 4 | 5 | 4 | 3 | 5 | 4 | 2 |
| Num. H-bond donors | 3 | 5 | 4 | 2 | 5 | 4 | 1 | 1 | 3 | 0 | 3 | 2 | 3 | 4 | 2 | 2 |
| Molar Refractivity | 64.37 | 61.65 | 74.31 | 74.43 | 133.54 | 132.38 | 80.94 | 44.86 | 36.08 | 83.25 | 58.86 | 60.34 | 84.06 | 128.18 | 53.73 | 102.42 |
| TPSA (Å2) | 78.43 | 158.4 | 129.72 | 83.63 | 101.15 | 80.92 | 37.3 | 55.4 | 52.49 | 60.44 | 92.78 | 74.6 | 66.48 | 97.99 | 74.6 | 49.33 |
| Consensus Log Po/w | 0.04 | 3.41 | −0.23 | 0.32 | 3.27 | 4.66 | 5.59 | 0.88 | −0.07 | 2.9 | −0.12 | 1.23 | 3.64 | 3.74 | 1.88 | 5.5 |
| Lipinski’s Rule | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Bioavailability Score | 0.55 | 0.55 | 0.56 | 0.55 | 0.55 | 0.55 | 0.56 | 0.55 | 0.55 | 0.55 | 0.55 | 0.56 | 0.55 | 0.56 | 0.56 | 0.55 |
| Pharmacokinetics | ||||||||||||||||
| GI absorption | High | High | High | High | High | High | High | High | High | High | High | High | High | High | High | High |
| BBB permeant | No | Yes | No | No | No | No | No | No | No | Yes | No | No | Yes | No | Yes | Yes |
| P-gp substrate | Yes | No | No | No | Yes | Yes | No | No | No | No | No | No | Yes | Yes | No | No |
| CYP1A2 inhibitor | No | Yes | No | No | No | No | Yes | No | No | No | No | No | No | No | No | Yes |
| CYP2C19 inhibitor | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| CYP2C9 inhibitor | No | No | No | No | No | No | Yes | No | No | No | No | No | No | No | No | No |
| CYP2D6 inhibitor | No | No | No | No | No | No | No | No | No | No | No | No | Yes | No | No | No |
| CYP3A4 inhibitor | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| Log Kp (cm/s) | −9.4 | −4.8 | −10.23 | −8.87 | −7.12 | −4.56 | −2.64 | −6.75 | −7.69 | −6.51 | −9.05 | −7.41 | −4.62 | −6.44 | −6.04 | −3.14 |
| Allium subhirsutum Methanolic Extract | |||
|---|---|---|---|
| Phytochemical Composition | Antioxidant Activities | ||
| TPC (mg GAE/g Extract) | TFC (mg QE/g Extract) | Reducing Power IC50 (mg/mL) | DPPH IC50 (mg/mL) |
| 17.6 ± 0.8 | 5.5 ± 0.8 | 0.45 ± 0.02 | 24.3 ± 1.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badraoui, R.; Rebai, T.; Elkahoui, S.; Alreshidi, M.; N. Veettil, V.; Noumi, E.; A. Al-Motair, K.; Aouadi, K.; Kadri, A.; De Feo, V.; et al. Allium subhirsutum L. as a Potential Source of Antioxidant and Anticancer Bioactive Molecules: HR-LCMS Phytochemical Profiling, In Vitro and In Vivo Pharmacological Study. Antioxidants 2020, 9, 1003. https://doi.org/10.3390/antiox9101003
Badraoui R, Rebai T, Elkahoui S, Alreshidi M, N. Veettil V, Noumi E, A. Al-Motair K, Aouadi K, Kadri A, De Feo V, et al. Allium subhirsutum L. as a Potential Source of Antioxidant and Anticancer Bioactive Molecules: HR-LCMS Phytochemical Profiling, In Vitro and In Vivo Pharmacological Study. Antioxidants. 2020; 9(10):1003. https://doi.org/10.3390/antiox9101003
Chicago/Turabian StyleBadraoui, Riadh, Tarek Rebai, Salem Elkahoui, Mousa Alreshidi, Vajid N. Veettil, Emira Noumi, Khaled A. Al-Motair, Kaïss Aouadi, Adel Kadri, Vincenzo De Feo, and et al. 2020. "Allium subhirsutum L. as a Potential Source of Antioxidant and Anticancer Bioactive Molecules: HR-LCMS Phytochemical Profiling, In Vitro and In Vivo Pharmacological Study" Antioxidants 9, no. 10: 1003. https://doi.org/10.3390/antiox9101003