Conservation of Native Wild Ivory-White Olives from the MEDES Islands Natural Reserve to Maintain Virgin Olive Oil Diversity
Abstract
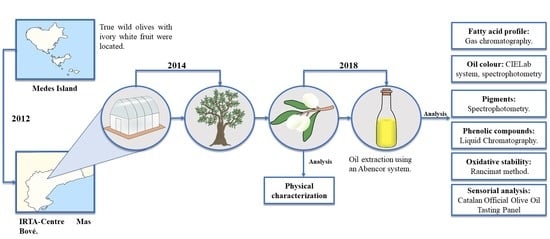
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Olive Oil Extraction
2.4. Olive Oil Characterization
2.4.1. Fatty Acid Composition
2.4.2. Pigments and Color
2.4.3. Phenolic Compounds
2.4.4. Oxidative Stability
2.4.5. Sensorial Analysis
3. Results and Discussion
3.1. Physical Characterization
3.2. Fatty Acid Profile
3.3. Pigments and Color
3.4. Phenolic Compounds
3.5. Oxidative Stability
3.6. Sensorial Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Williams, D.R.; Balmford, A.; Wilcove, D.S. The past and future role of conservation science in saving biodiversity. Conserv. Lett. 2020, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kitzes, J.; Berlow, E.; Conlisk, E.; Erb, K.; Iha, K.; Martinez, N.; Newman, E.A.; Plutzar, C.; Smith, A.B.; Harte, J. Consumption-based conservation targeting: Linking biodiversity loss to upstream demand through a global wildlife footprint. Conserv. Lett. 2017, 10, 531–538. [Google Scholar] [CrossRef]
- Dudley, N. Guidelines for Applying Protected Area Management Categories; IUCN: Gland, Switzerland, 2008. [Google Scholar]
- De la Guerra, M.M.; Alandi, C.M.; Blázquez, J.P.; Mezquida, J.A.A.; García, J.G.-L.; Ventura, D.G. Anuario 2018 del Estado de Las Áreas Protegidas en España; EUROPARC-España: Madrid, Spain, 2018. [Google Scholar]
- Ros, J. The Medes isles, paradise around the corner. Catalonia 1992, 31, 20–23. [Google Scholar]
- Julca, I.; Marcet-Houben, M.; Cruz, F.; Gomez-Garrido, J.; Gaut, B.S.; Diez, C.M.; Gut, I.G.; Alioto, T.S.; Vargas, P.; Gabaldon, T. Genomic evidence for recurrent genetic admixture during domestication mediterranean olive trees (Olea europaea). bioRxiv 2020. [Google Scholar] [CrossRef]
- Green, P.S. A revision of Olea L. (Oleaceae). KEW Bull. 2002, 57, 91–140. [Google Scholar] [CrossRef]
- Lumaret, R.; Ouazzani, N. Ancient wild olives in Mediterranean forests. Nature 2001, 413, 700. [Google Scholar] [CrossRef]
- Nichols, J.D.; Williams, B.K. Monitoring for conservation. Trends Ecol. Evol. 2006, 21, 668–673. [Google Scholar] [CrossRef]
- Belaj, A.; Muñoz-Diez, C.; Baldoni, L.; Satovic, Z.; Barranco, D. Genetic diversity and relationships of wild and cultivated olives at regional level in Spain. Sci. Hortic. 2010, 124, 323–330. [Google Scholar] [CrossRef]
- Belaj, A.; Muñoz-Diez, C.; Baldoni, L.; Porceddu, A.; Barranco, D.; Satovic, Z. Genetic diversity and population structure of wild olives from the north-western Mediterranean assessed by SSR markers. Ann. Bot. 2007, 100, 449–458. [Google Scholar] [CrossRef] [Green Version]
- Besnard, G.; Hernández, P.; Khadari, B.; Dorado, G.; Savolainen, V. Genomic profiling of plastid DNA variation in the Mediterranean olive tree. BMC Plant. Biol. 2011, 11, 80. [Google Scholar] [CrossRef] [Green Version]
- Cirilli, M.; Bellincontro, A.; Urbani, S.; Servili, M.; Esposto, S.; Mencarelli, F.; Muleo, R. On-field monitoring of fruit ripening evolution and quality parameters in olive mutants using a portable NIR-AOTF device. Food Chem. 2016, 199, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Pasqualone, A.; Di Rienzo, V.; Blanco, A.; Summo, C.; Caponio, F.; Montemurro, C. Characterization of virgin olive oil from Leucocarpa cultivar by chemical and DNA analysis. Food Res. Int. 2012, 47, 188–193. [Google Scholar] [CrossRef]
- Tous, J.; Romero, A. Marfil’ olive. HortScience 1998, 33, 162–163. [Google Scholar] [CrossRef] [Green Version]
- Lia, F.; Zammit-Mangion, M.; Farrugia, C. A first description of the phenolic profile of EVOOs from the Maltese islands using SPE and HPLC: Pedo-climatic conditions modulate genetic factors. Agriculture 2019, 9, 107. [Google Scholar] [CrossRef] [Green Version]
- Yubero-Serrano, E.M.; Lopez-Moreno, J.; Gomez-Delgado, F.; Lopez-Miranda, J. Extra virgin olive oil: More than a healthy fat. Eur. J. Clin. Nutr. 2019, 72, 8–17. [Google Scholar] [CrossRef] [Green Version]
- León, L.; De La Rosa, R.; Velasco, L.; Belaj, A. Using wild olives in breeding programs: Implications on oil quality composition. Front. Plant Sci. 2018, 9, 232. [Google Scholar] [CrossRef]
- Hannachi, H.; Breton, C.; Msallem, M.; El Hadj, S.B.; El Gazzah, M.; Berville, A. Differences between native and introduced olive cultivars as revealed by morphology of drupes, oil composition and SSR polymorphisms: A case study in Tunisia. Sci. Hortic. 2008, 116, 280–290. [Google Scholar] [CrossRef]
- Di Vaio, C.; Nocerino, S.; Paduano, A.; Sacchi, R. Influence of some environmental factors on drupe maturation and olive oil composition. J. Sci. Food Agric. 2013, 93, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Rotondi, A.; Alfei, B.; Magli, M.; Pannelli, G. Influence of genetic matrix and crop year on chemical and sensory profiles of Italian monovarietal extra-virgin olive oils. J. Sci. Food Agric. 2010, 90, 2641–2648. [Google Scholar] [CrossRef]
- Salazar-García, D.C.; Malheiro, R.; Pereira, J.A.; Lopéz-Cortés, I. Unexplored olive cultivars from the Valencian Community (Spain): Some chemical characteristics as a valorization strategy. Eur. Food Res. Technol. 2019, 245, 325–334. [Google Scholar] [CrossRef] [Green Version]
- Fratianni, F.; Cozzolino, R.; Martignetti, A.; Malorni, L.; d’Acierno, A.; De Feo, V.; da Cruz, A.G.; Nazzaro, F. Biochemical composition and antioxidant activity of three extra virgin olive oils from the Irpinia Province, Southern Italy. Food Sci. Nutr. 2019, 7, 3233–3243. [Google Scholar] [CrossRef]
- Uceda, M.; Frías, L. Seasons of harvest. Changes on fruit oil content, oil composition and oil quality. In Proceedings of the II Seminario Oleicola International. IOOC, Cordoba, Spain, 1975; pp. 25–46. [Google Scholar]
- Morrison, W.R.; Smith, L.M. Preparation of fatty acid methyl esters and dimethylacetals from lipids. J. Lipid Res. 1964, 5, 600–608. [Google Scholar]
- Minguez-Mosquera, I.M.; Rejano-Navarro, L.; Gandul-Rojas, B.; SanchezGomez, A.H.; Garrido-Fernandez, J. Color-pigment correlation in virgin olive oil. J. Am. Oil Chem. Soc. 1991, 68, 332–336. [Google Scholar] [CrossRef]
- Lopez-Yerena, A.; Lozano-Castellon, J.; Olmo-Cunillera, A.; Tresserra-Rimbau, A.; Quifer-Rada, P.; Jiménez, B.; Perez, M.; Vallverdu-Queralt, A. Effects of organic and conventional growing systems on the phenolic profile of extra-virgin olive oil. Molecules. 2019, 24, 1986. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez Rosales, F. Determinación de la estabilidad oxidativa de aceites de oliva vírgenes: Comparación entre el método del oxígeno activo (AOM) y el método rancimat. Grasas y Aceites. 1989, 40, 1–5. [Google Scholar]
- Commission Regulation (EEC). On the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis; European Comission: Brussels, Belgium, 1991. [Google Scholar]
- Hannachi, H.; Nasri, N.; Elfalleh, W.; Tlili, N.; Ferchichi, A.; Msallem, M. Fatty acids, sterols, polyphenols, and chlorophylls of olive oils obtained from Tunisian wild olive trees (Olea europaea L. Var Sylvestris). Int. J. Food Prop. 2013, 16, 1271–1283. [Google Scholar] [CrossRef]
- Belaj, A.; Leon, L.; Satovic, Z.; de la Rosa, R. Variability of wild olives (Olea europaea Subsp. europaea Var. Sylvestris) analyzed by agro-morphological traits and SSR markers. Sci. Hortic. 2011, 129, 561–569. [Google Scholar] [CrossRef]
- Conte, P.; Squeo, G.; Difonzo, G.; Caponio, F.; Fadda, C.; Del Caro, A.; Urgeghe, P.P.; Montanari, L.; Montinaro, A.; Piga, A. Change in quality during ripening of olive fruits and related oils extracted from three minor autochthonous Sardinian cultivars. Emir. J. Food Agric. 2019, 31, 196–205. [Google Scholar] [CrossRef]
- Beltran, G.; del Rio, C.; Sanchez, S.; Martinez, L. Influence of harvest date and crop yield on the fatty acid composition of virgin olive oils from Cv. Picual. J. Agric. Food Chem. 2004, 52, 3434–3440. [Google Scholar] [CrossRef] [PubMed]
- Baccouri, B.; Guerfel, M.; Zarrouk, W.; Taamalli, W.; Daoud, D.; Zarrouk, M. Wild olive (Olea Europaea L.) selection for quality oil production. J. Food Biochem. 2011, 35, 161–176. [Google Scholar] [CrossRef]
- Ozcan, M.M.; Al Juhaimi, F.; Uslu, N.; Ghafoor, K.; Mohamed Ahmed, I.A.; Babiker, E.E. The effect of olive varieties on fatty acid composition and tocopherol contents of cold pressed virgin olive oils. J. Oleo Sci. 2019, 68, 307–310. [Google Scholar] [CrossRef] [Green Version]
- Aguilera, M.P.; Beltran, G.; Ortega, D.; Fernandez, A.; Jimenez, A.; Uceda, M. Characterisation of virgin olive oil of Italian olive cultivars: “Frantoio” and “Leccino”, grown in Andalusia. Food Chem. 2005, 89, 387–391. [Google Scholar] [CrossRef]
- Borges, T.H.; Pereira, J.A.; Cabrera-Vique, C.; Lara, L.; Oliveira, A.F.; Seiquer, I. Characterization of Arbequina virgin olive oils produced in different regions of Brazil and Spain: Physicochemical properties, oxidative stability and fatty acid profile. Food Chem. 2017, 215, 454–462. [Google Scholar] [CrossRef]
- Yu, L.; Wang, Y.; Wu, G.; Jin, J.; Jin, Q.; Wang, X. Quality and composition of virgin olive oils from indigenous and European cultivars grown in China. JAOCS J. Am. Oil Chem. Soc. 2019, 97, 341–353. [Google Scholar] [CrossRef]
- Salas, J.J.; Sanchez, J.; Ramli, U.S.; Manaf, A.M.; Williams, M.; Harwood, J.L. Biochemistry of lipid metabolism in olive and other oil fruits. Prog. Lipid Res. 2000, 39, 151–180. [Google Scholar] [CrossRef]
- Al-Bachir, M.; Sahloul, H. Fatty acid profile of olive oil extracted from irradiated and non-irradiated olive fruits. Int. J. Food Prop. 2017, 20, 2550–2558. [Google Scholar] [CrossRef] [Green Version]
- Dag, A.; Kerem, Z.; Yogev, N.; Zipori, I.; Lavee, S.; Ben-David, E. Influence of time of harvest and maturity index on olive oil yield and quality. Sci. Hortic. 2011, 127, 358–366. [Google Scholar] [CrossRef]
- Reboredo-Rodriguez, P.; Gonzalez-Barreiro, C.; Cancho-Grande, B.; Fregapane, G.; Salvador, M.D.; Simal-Gandara, J. Characterisation of extra virgin olive oils from Galician autochthonous varieties and their co-crushings with Arbequina and Picual cv. Food Chem. 2015, 176, 493–503. [Google Scholar] [CrossRef]
- Allalout, A.; Krichene, D.; Methenni, K.; Taamalli, A.; Oueslati, I.; Daoud, D.; Zarrouk, M. Characterization of virgin olive oil from super intensive spanish and greek varieties grown in northern Tunisia. Sci. Hortic. 2009, 120, 77–83. [Google Scholar] [CrossRef]
- Criado, M.N.; Romero, M.P.; Casanovas, M.; Motilva, M.J. Pigment profile and colour of monovarietal virgin olive oils from Arbequina cultivar obtained during two consecutive crop seasons. Food Chem. 2008, 110, 873–880. [Google Scholar] [CrossRef]
- Giuffrida, D.; Salvo, F.; Salvo, A.; La Pera, L.; Dugo, G. Pigments composition in monovarietal virgin olive oils from various sicilian olive varieties. Food Chem. 2007, 101, 833–837. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Badeka, A.; Casiello, G.; Longobardi, F.; Kontominas, M.G. Rapid screening of olive oil cultivar differentiation based on selected physicochemical parameters, pigment content and fatty acid composition using advanced chemometrics. Eur. Food Res. Technol. 2019, 245, 2027–2038. [Google Scholar] [CrossRef]
- Dabbou, S.; Brahmi, F.; Taamali, A.; Issaoui, M.; Ouni, Y.; Braham, M.; Zarrouk, M.; Hammami, M. Extra virgin olive oil components and oxidative stability from olives grown in Tunisia. JAOCS J. Am. Oil Chem. Soc. 2010, 87, 1199–1209. [Google Scholar] [CrossRef]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health effects of phenolic compounds found in extra-virgin olive oil, by-products, and leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef] [Green Version]
- Juliano, P.; Bainczyk, F.; Swiergon, P.; Supriyatna, M.I.M.; Guillaume, C.; Ravetti, L.; Canamasas, P.; Cravotto, G.; Xu, X.Q. Extraction of olive oil assisted by high-frequency ultrasound standing waves. Ultrason. Sonochemistry 2017, 38, 104–114. [Google Scholar] [CrossRef]
- Taticchi, A.; Selvaggini, R.; Esposto, S.; Sordini, B.; Veneziani, G.; Servili, M. Physicochemical characterization of virgin olive oil obtained using an ultrasound-assisted extraction at an industrial scale: Influence of olive maturity index and malaxation time. Food Chem. 2019, 289, 7–15. [Google Scholar] [CrossRef]
- Bouarroudj, K.; Tamendjari, A.; Larbat, R. Quality, composition and antioxidant activity of Algerian wild olive (Olea Europaea L. Subsp. Oleaster) oil. Ind. Crop. Prod. 2016, 83, 484–491. [Google Scholar] [CrossRef]
- Celano, R.; Piccinelli, A.L.; Pugliese, A.; Carabetta, S.; Di Sanzo, R.; Rastrelli, L.; Russo, M. Insights into the analysis of phenolic secoiridoids in extra virgin olive oil. J. Agric. Food Chem. 2018, 66, 6053–6063. [Google Scholar] [CrossRef]
- Lozano-Castellon, J.; Lopez-Yerena, A.; Rinaldi de Alvarenga, J.F.; Romero del Castillo-Alba, J.; Vallverdu-Queralt, A.; Escribano-Ferrer, E.; Lamuela-Raventos, R.M. Health-promoting properties of oleocanthal and oleacein: Two secoiridoids from extra-virgin olive oil. Crit. Rev. Food Sci. Nutr. 2019, 60, 2532–2548. [Google Scholar] [CrossRef]
- Francisco, V.; Ruiz-Fernandez, C.; Lahera, V.; Lago, F.; Pino, J.; Skaltsounis, L.; Gonzalez-Gay, M.A.; Mobasheri, A.; Gómez, R.; Scotece, M.; et al. Natural molecules for healthy lifestyles: Oleocanthal from extra virgin olive oil. J. Agric. Food Chem. 2019, 67, 3845–3853. [Google Scholar] [CrossRef]
- Karkoula, E.; Skantzari, A.; Melliou, E.; Magiatis, P. Direct measurement of oleocanthal and oleacein levels in olive oil by quantitative1H NMR. Establishment of a new index for the characterization of extra virgin olive oils. J. Agric. Food Chem. 2012, 60, 11696–11703. [Google Scholar] [CrossRef]
- Miho, H.; Diez, C.M.; Mena-Bravo, A.; Sanchez de Medina, V.; Moral, J.; Melliou, E.; Magiatis, P.; Rallo, L.; Barranco, D.; Priego-Capote, F. Cultivar influence on variability in olive oil phenolic profiles determined through an extensive germplasm survey. Food Chem. 2018, 266, 192–199. [Google Scholar] [CrossRef]
- Deiana, P.; Santona, M.; Dettori, S.; Culeddu, N.; Dore, A.; Molinu, M.G. Multivariate approach to assess the chemical composition of Italian virgin olive oils as a function of variety and harvest period. Food Chem. 2019, 300, 125243. [Google Scholar] [CrossRef]
- EFSA. Scientific opinion on the substantiation of a health claim related to polyphenols in olive and maintenance of normal blood HDL cholesterol concentrations (ID 1639, further assessment) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2012, 10, 2848. [Google Scholar] [CrossRef] [Green Version]
- Lanza, B.; Di Marco, S.; Simone, N.; Di Marco, C.; Gabriele, F. Table olives fermented in iodized sea salt brines: Nutraceutical/sensory properties and microbial biodiversity. Foods 2020, 9, 301. [Google Scholar] [CrossRef] [Green Version]
- Lanza, B.; Amoruso, F.; Di Serio, M.G. Nutritional composition and kinaesthetic properties of white table olives from Olea europaea L. Leucocarpa Cultivar. Rivista Italiana delle Sostanze Grasse 2016, 93, 157–164. [Google Scholar]
- de Torres, A.; Espinola, F.; Moya, M.; Alcala, S.; Vidal, A.M.; Castro, E. Assessment of phenolic compounds in virgin olive oil by response surface methodology with particular focus on flavonoids and lignans. LWT - Food Sci. Technol. 2018, 90, 22–30. [Google Scholar] [CrossRef]
- Negro, C.; Aprile, A.; Luvisi, A.; Nicoli, F.; Nutricati, E.; Vergine, M.; Miceli, A.; Blando, F.; Sabella, E.; De Bellis, L. Phenolic profile and antioxidant activity of Italian Monovarietal extra virgin olive oils. Antioxidants 2019, 8, 161. [Google Scholar] [CrossRef] [Green Version]
- Aparicio, R.; Roda, L.; Albi, M.A.; Gutierrez, F. Effect of various compounds on virgin olive oil stability measured by Rancimat. J. Agric. Food Chem. 1999, 47, 4150–4155. [Google Scholar] [CrossRef]
- Tous, J.; Romero, A.; Francisco, J.; Ninot, A. Mediterranean clonal selections evaluated for modern hedgerow olive oil production in Spain. Calif. Agric. 2011, 65, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Velasco, J.; Dobarganes, C. Oxidative stability of virgin olive oil. Eur. J. Lipid Sci. Technol. 2002, 104, 661–676. [Google Scholar] [CrossRef]
- Bradley, D.G.; Min, D.B. Singlet oxygen oxidation of foods. Crit. Rev. Food Sci. Nutr. 1992, 31, 211–236. [Google Scholar] [CrossRef]
- Baldioli, M.; Servili, M.; Perretti, G.; Montedoro, G.F. Antioxidant activity of tocopherols and phenolic compounds of virgin olive oil. J. Am. Oil Chem. Soc. 1996, 73, 1589–1593. [Google Scholar] [CrossRef]
- Franco, M.N.; Galeano-Diaz, T.; Sanchez, J.; De Miguel, C.; Martin-Vertedor, D. Antioxidant capacity of the phenolic fraction and its effect on the oxidative stability of olive oil varieties grown in the southwest of Spain. J. Oleo Sci. 2014, 65, 004. [Google Scholar]
- Bate-Smith, E.C. Haemanalysis of tannins: The concept of relative astringency. Phytochemistry 1973, 12, 907–912. [Google Scholar] [CrossRef]
- Whitehead, M.C.; Beeman, C.S.; Kinsella, B.A. Distribution of taste and general sensory nerve endings in fungiform papillae of the hamster. Am. J. Anat. 1985, 173, 185–201. [Google Scholar] [CrossRef]
- Reboredo-Rodriguez, P.; Gonzaalez-Barreiro, C.; Cancho-Grande, B.; Simal-Gaandara, J. Concentrations of aroma compounds and odor activity values of odorant series in different olive cultivars and their oils. J. Agric. Food Chem. 2013, 61, 5252–5259. [Google Scholar] [CrossRef]
- Morales, M.T.; Rios, J.J.; Aparicio, R. Changes in the volatile composition of virgin olive oil during oxidation: Flavors and off-flavors. J. Agric. Food Chem. 1997, 45, 2666–2673. [Google Scholar] [CrossRef]

| Physical Characteristics | Chromatic Parameters | Pigments (mg/kg) | |||
|---|---|---|---|---|---|
| Fruit weight (g) | 0.78 ± 0.08 | L* | 88.6 ± 6.7 | Chlorophylls | 12.5 ± 4.6 |
| Flesh weight (g) | 0.57 ± 0.06 | a* | −9.3 ± 1.4 | Carotenoids | 9.2 ± 3.3 |
| Pit weight (g) | 0.21 ± 0.03 | b* | 83.7 ± 23.3 | Chlorophylls/carotenoids | 1.4 ± 1.4 |
| Fat content (%) | 28.22 ± 3.91 | Chroma | 84.2 ± 23.3 | Oxidative Stability (h at 120 °C) | |
| Ripening index | 3.18 ± 0.42 | a*/b* | −0.1 ± 0.06 | Stability | 15.8 ± 5.9 |
| Fatty Acid Composition (%) | |||
|---|---|---|---|
| Palmitic | 20.11 ± 2.46 | Fatty acid indices | |
| Palmitoleic | 3.51 ± 0.96 | MUFA (%) | 62.51 ± 6.46 |
| Margaric | 0.39 ± 0.37 | PUFA (%) | 14.90 ± 4.20 |
| Stearic | 1.79 ± 0.07 | MUFA / PUFA | 4.20 ± 2.60 |
| Oleic | 58.80 ± 7.05 | C18:1 / C18:2 | 4.36 ± 3.16 |
| Linoleic | 13.48 ± 4.09 | SFA (%) | 22.60 ± 2.48 |
| Linolenic | 1.28 ± 0.18 | UFA / SFA | 3.43 ± 0.53 |
| Arachidic | 0.30 ± 0.02 | ||
| Eicosenoic | 0.20 ± 0.04 | ||
| Eicosadienoic | 0.14 ± 0.13 | ||
| Phenolic Compounds (mg/kg) | |
|---|---|
| Total phenols | 669.97 ± 119.38 |
| Oleacein | 373.29 ± 72.02 |
| Oleocanthal | 204.84 ± 52.58 |
| Oleuropein aglycone | 33.82 ± 12.61 |
| Ligstroside aglycone | 14.15 ± 3.34 |
| Elenolic acid | 17.18 ± 9.47 |
| Hydroxytyrosol | 0.77 ± 0.57 |
| Hydroxyelenolic acid | 0.27 ± 0.19 |
| Lactone | 4.94 ± 1.77 |
| HDCM-OA | 0.37 ± 0.18 |
| Luteolin | 18.76 ± 10.77 |
| Apigenin | 1.56 ± 0.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Yerena, A.; Ninot, A.; Lozano-Castellón, J.; Escribano-Ferrer, E.; Romero-Aroca, A.J.; Belaj, A.; Vallverdú-Queralt, A.; Lamuela-Raventós, R.M. Conservation of Native Wild Ivory-White Olives from the MEDES Islands Natural Reserve to Maintain Virgin Olive Oil Diversity. Antioxidants 2020, 9, 1009. https://doi.org/10.3390/antiox9101009
López-Yerena A, Ninot A, Lozano-Castellón J, Escribano-Ferrer E, Romero-Aroca AJ, Belaj A, Vallverdú-Queralt A, Lamuela-Raventós RM. Conservation of Native Wild Ivory-White Olives from the MEDES Islands Natural Reserve to Maintain Virgin Olive Oil Diversity. Antioxidants. 2020; 9(10):1009. https://doi.org/10.3390/antiox9101009
Chicago/Turabian StyleLópez-Yerena, Anallely, Antònia Ninot, Julián Lozano-Castellón, Elvira Escribano-Ferrer, Agustí J. Romero-Aroca, Angjelina Belaj, Anna Vallverdú-Queralt, and Rosa M. Lamuela-Raventós. 2020. "Conservation of Native Wild Ivory-White Olives from the MEDES Islands Natural Reserve to Maintain Virgin Olive Oil Diversity" Antioxidants 9, no. 10: 1009. https://doi.org/10.3390/antiox9101009