Abstract
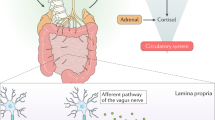
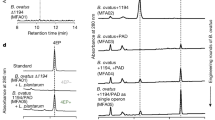
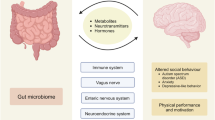
Mounting evidence suggests that the gut microbiome impacts brain development and function. Gut–brain connections may be mediated by an assortment of microbial molecules that are produced in the gastrointestinal tract, which can subsequently permeate many organs, including sometimes the brain. Studies in animal models have identified molecular cues propagated from intestinal bacteria to the brain that can affect neurological function and/or neurodevelopmental and neurodegenerative conditions. Herein, we describe bacterial metabolites with known or suspected neuromodulatory activity, define mechanisms of signalling pathways from the gut microbiota to the brain and discuss direct effects that gut bacterial molecules are likely exerting on specific brain cells. Many discoveries are recent, and the findings described in this Perspective are largely novel and yet to be extensively validated. However, expanding research into the dynamic molecular communications between gut microorganisms and the CNS continues to uncover critical and previously unappreciated clues in understanding the pathophysiology of behavioural, psychiatric and neurodegenerative diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cho, I. & Blaser, M. J. The human microbiome: at the interface of health and disease. Nat. Rev. Genet. 13, 260–270 (2012).
Koppel, N., Rekdal, V. M. & Balskus, E. P. Chemical transformation of xenobiotics by the human gut microbiota. Science 356, eaag2770 (2017).
Sonnenburg, J. L. & Bäckhed, F. Diet–microbiota interactions as moderators of human metabolism. Nature 535, 56–64 (2016).
Nyangahu, D. D. & Jaspan, H. B. Influence of maternal microbiota during pregnancy on infant immunity. Clin. Exp. Immunol. 198, 47–56 (2019).
Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214 (2012).
Kagnoff, M. F. Immunology of the intestinal tract. Gastroenterology 105, 1275–1280 (1993).
Furness, J. B., Callaghan, B. P., Rivera, L. R. & Cho, H.-J. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv. Exp. Med. Biol. 817, 39–71 (2014).
Clarke, G. et al. The microbiome–gut–brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 18, 666–673 (2013).
Neufeld, K. M., Kang, N., Bienenstock, J. & Foster, J. A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 23, 255–e119 (2011).
Fröhlich, E. E. et al. Cognitive impairment by antibiotic-induced gut dysbiosis: analysis of gut microbiota–brain communication. Brain Behav. Immun. 56, 140–155 (2016).
Desbonnet, L. et al. Gut microbiota depletion from early adolescence in mice: implications for brain and behaviour. Brain Behav. Immun. 48, 165–173 (2015).
Diaz Heijtz, R. et al. Normal gut microbiota modulates brain development and behavior. Proc. Natl Acad. Sci. USA 108, 3047–3052 (2011).
Matsumoto, M. et al. Cerebral low-molecular metabolites influenced by intestinal microbiota: a pilot study. Front. Syst. Neurosci. 7, 9 (2013).
Sudo, N. et al. Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J. Physiol. 558, 263–275 (2004).
Savignac, H. M. et al. Prebiotic feeding elevates central brain derived neurotrophic factor, N-methyl-D-aspartate receptor subunits and D-serine. Neurochem. Int. 63, 756–764 (2013).
Bora, S. A., Kennett, M. J., Smith, P. B., Patterson, A. D. & Cantorna, M. T. The gut microbiota regulates endocrine vitamin D metabolism through fibroblast growth factor 23. Front. Immunol. 9, 408 (2018).
Monteggia, L. M. et al. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc. Natl Acad. Sci. USA 101, 10827–10832 (2004).
Luczynski, P. et al. Adult microbiota-deficient mice have distinct dendritic morphological changes: differential effects in the amygdala and hippocampus. Eur. J. Neurosci. 44, 2654–2666 (2016).
Lu, J. et al. Microbiota influence the development of the brain and behaviors in C57BL/6J mice. PLoS ONE 13, e0201829 (2018).
Hoban, A. E. et al. Regulation of prefrontal cortex myelination by the microbiota. Transl Psychiatry 6, e774 (2016).
Hoban, A. E. et al. The microbiome regulates amygdala-dependent fear recall. Mol. Psychiatry 23, 1134–1144 (2018).
Desbonnet, L., Clarke, G., Shanahan, F., Dinan, T. G. & Cryan, J. F. Microbiota is essential for social development in the mouse. Mol. Psychiatry 19, 146–148 (2014).
Buffington, S. A. et al. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell 165, 1762–1775 (2016).
Leclercq, S. et al. Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nat. Commun. 8, 15062 (2017).
Luo, Y. et al. Gut microbiota regulates mouse behaviors through glucocorticoid receptor pathway genes in the hippocampus. Transl. Psychiatry 8, 187 (2018).
Huo, R. et al. Microbiota modulate anxiety-like behavior and endocrine abnormalities in hypothalamic–pituitary–adrenal axis. Front. Cell. Infect. Microbiol. 7, 489 (2017).
Lukić, I., Getselter, D., Koren, O. & Elliott, E. Role of tryptophan in microbiota-induced depressive-like behavior: evidence from tryptophan depletion study. Front. Behav. Neurosci. 13, 123 (2019).
Ceylani, T., Jakubowska-Doğru, E., Gurbanov, R., Teker, H. T. & Gozen, A. G. The effects of repeated antibiotic administration to juvenile BALB/c mice on the microbiota status and animal behavior at the adult age. Heliyon 4, e00644 (2018).
Zhai, B., Shang, X., Fu, J., Li, F. & Zhang, T. Rapamycin relieves anxious emotion and synaptic plasticity deficits induced by hindlimb unloading in mice. Neurosci. Lett. 677, 44–48 (2018).
Hoban, A. E. et al. Behavioural and neurochemical consequences of chronic gut microbiota depletion during adulthood in the rat. Neuroscience 339, 463–477 (2016).
Wang, B., Yao, M., Lv, L., Ling, Z. & Li, L. The human microbiota in health and disease. Engineering 3, 71–82 (2017).
Kang, D.-W. et al. Differences in fecal microbial metabolites and microbiota of children with autism spectrum disorders. Anaerobe 49, 121–131 (2018).
Zheng, P. et al. The gut microbiome from patients with schizophrenia modulates the glutamate–glutamine–GABA cycle and schizophrenia-relevant behaviors in mice. Sci. Adv. 5, eaau8317 (2019).
Jiang, H. et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 48, 186–194 (2015).
Prehn-Kristensen, A. et al. Reduced microbiome alpha diversity in young patients with ADHD. PLoS ONE 13, e0200728 (2018).
Vogt, N. M. et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 7, 1–11 (2017).
Scheperjans, F. et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord. 30, 350–358 (2015).
Jangi, S. et al. Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 7, 12015 (2016).
Berer, K. et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479, 538–541 (2011).
Sampson, T. R. et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167, 1469–1480.e12 (2016).
Fujii, Y. et al. Fecal metabolite of a gnotobiotic mouse transplanted with gut microbiota from a patient with Alzheimer’s disease. Biosci. Biotechnol. Biochem. https://doi.org/10.1080/09168451.2019.1644149 (2019).
Zheng, P. et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 21, 786–796 (2016).
Tengeler, A. C. et al. Gut microbiota from persons with attention-deficit/hyperactivity disorder affects the brain in mice. Microbiome 8, 44 (2020).
Sharon, G. et al. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell 177, 1600–1618 (2019).
Walter, J., Armet, A. M., Finlay, B. B. & Shanahan, F. Establishing or exaggerating causality for the gut microbiome: lessons from human microbiota-associated rodents. Cell 180, 221–232 (2020).
Hsiao, E. Y. et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155, 1451–1463 (2013).
Tabouy, L. et al. Dysbiosis of microbiome and probiotic treatment in a genetic model of autism spectrum disorders. Brain Behav. Immun. 73, 310–319 (2018).
Ochoa-Repáraz, J. et al. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J. Immunol. 183, 6041–6050 (2009).
Seifert, H. A. et al. Antibiotics protect against EAE by increasing regulatory and anti-inflammatory cells. Metab. Brain Dis. 33, 1599–1607 (2018).
He, B. et al. Lactobacillus reuteri reduces the severity of experimental autoimmune encephalomyelitis in mice by modulating gut microbiota. Front Immunol 10, 384 (2019).
Bravo, J. A. et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl Acad. Sci. USA 108, 16050–16055 (2011).
Liu, W.-H. et al. Genome architecture of Lactobacillus plantarum PS128, a probiotic strain with potential immunomodulatory activity. Gut Pathog. 7, 22 (2015).
Burokas, A. et al. Targeting the microbiota–gut–brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol. Psychiatry 82, 472–487 (2017).
Lee, H.-J., Lee, K.-E., Kim, J.-K. & Kim, D.-H. Suppression of gut dysbiosis by Bifidobacterium longum alleviates cognitive decline in 5XFAD transgenic and aged mice. Sci. Rep. 9, 11814 (2019).
Li, Y. et al. Neuroprotective effects of intravenous transplantation of bone marrow mononuclear cells from 5-fluorouracil pre-treated rats on ischemic stroke. Behav. Brain Res. 301, 287–292 (2016).
Sandler, R. H. et al. Short-term benefit from oral vancomycin treatment of regressive-onset autism. J. Child. Neurol. 15, 429–435 (2000).
Kang, D.-W. et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome 5, 10 (2017).
Metz, L. M. et al. Trial of minocycline in a clinically isolated syndrome of multiple sclerosis. N. Engl. J. Med. 376, 2122–2133 (2017).
Allen, A. P. et al. Bifidobacterium longum 1714 as a translational psychobiotic: modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl. Psychiatry 6, e939 (2016).
Tillisch, K. et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology https://doi.org/10.1053/j.gastro.2013.02.043 (2013).
Steenbergen, L., Sellaro, R., van Hemert, S., Bosch, J. A. & Colzato, L. S. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun. 48, 258–264 (2015).
Messaoudi, M. et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 105, 755–764 (2011).
Bagga, D. et al. Probiotics drive gut microbiome triggering emotional brain signatures. Gut Microbes 9, 486–496 (2018).
Bagga, D. et al. Influence of 4-week multi-strain probiotic administration on resting-state functional connectivity in healthy volunteers. Eur. J. Nutr. 58, 1821–1827 (2019).
Vuong, H. E., Yano, J. M., Fung, T. C. & Hsiao, E. Y. The microbiome and host behavior. Annu. Rev. Neurosci. 40, 21–49 (2017).
Tierney, B. T. et al. The landscape of genetic content in the gut and oral human microbiome. Cell Host Microbe 26, 283–295.e8 (2019).
Meganathan, R. & Kwon, O. Biosynthesis of menaquinone (vitamin K2) and ubiquinone (coenzyme Q). EcoSal Plus https://doi.org/10.1128/ecosalplus.3.6.3.3 (2009).
Hanke, M. L. & Kielian, T. Toll-like receptors in health and disease in the brain: mechanisms and therapeutic potential. Clin. Sci. 121, 367–387 (2011).
Sellge, G. & Kufer, T. A. PRR-signaling pathways: learning from microbial tactics. Semin. Immunol. 27, 75–84 (2015).
Skaper, S. D., Facci, L., Zusso, M. & Giusti, P. An inflammation-centric view of neurological disease: beyond the neuron. Front. Cell Neurosci. 12, 72 (2018).
Arentsen, T. et al. The bacterial peptidoglycan-sensing molecule Pglyrp2 modulates brain development and behavior. Mol. Psychiatry 22, 257–266 (2017).
Vargas-Caraveo, A. et al. Lipopolysaccharide enters the rat brain by a lipoprotein-mediated transport mechanism in physiological conditions. Sci. Rep. 7, 13113 (2017).
Bassi, G. S. et al. Lipopolysaccharide-induced sickness behaviour evaluated in different models of anxiety and innate fear in rats. Basic Clin. Pharmacol. Toxicol. 110, 359–369 (2012).
Zhao, J. et al. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci. Rep. 9, 5790 (2019).
O’Connor, J. C. et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry 14, 511–522 (2009).
Romero, E. et al. Neurobehavioral and immunological consequences of prenatal immune activation in rats. Influence of antipsychotics. Neuropsychopharmacology 32, 1791–1804 (2007).
Izvolskaia, M., Sharova, V. & Zakharova, L. Prenatal programming of neuroendocrine system development by lipopolysaccharide: long-term effects. Int. J. Mol. Sci. 19, 3695 (2018).
Caputi, V. & Giron, M. C. Microbiome–gut–brain axis and Toll-like receptors in Parkinson’s disease. Int. J. Mol. Sci. 19, 1689 (2018).
Malkova, N. V., Yu, C. Z., Hsiao, E. Y., Moore, M. J. & Patterson, P. H. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav. Immun. 26, 607–616 (2012).
Ohgi, Y., Futamura, T., Kikuchi, T. & Hashimoto, K. Effects of antidepressants on alternations in serum cytokines and depressive-like behavior in mice after lipopolysaccharide administration. Pharmacol. Biochem. Behav. 103, 853–859 (2013).
Holzer, P. et al. Visceral inflammation and immune activation stress the brain. Front. Immunol. 8, 1613 (2017).
Popoff, M. R. & Poulain, B. Bacterial toxins and the nervous system: neurotoxins and multipotential toxins interacting with neuronal cells. Toxins 2, 683–737 (2010).
Kiu, R. & Hall, L. J. An update on the human and animal enteric pathogen Clostridium perfringens. Emerg. Microbes. Infect. https://doi.org/10.1038/s41426-018-0144-8 (2018).
Miyamoto, O. et al. Clostridium perfringens epsilon toxin causes excessive release of glutamate in the mouse hippocampus. FEMS Microbiol. Lett. 189, 109–113 (2000).
Yang, N. J. & Chiu, I. M. Bacterial signaling to the nervous system via toxins and metabolites. J. Mol. Biol. 429, 587–605 (2017).
Nagahama, M. & Sakurai, J. Distribution of labeled Clostridium perfringens epsilon toxin in mice. Toxicon 29, 211–217 (1991).
Agata, N., Ohta, M., Mori, M. & Isobe, M. A novel dodecadepsipeptide, cereulide, is an emetic toxin of Bacillus cereus. FEMS Microbiol. Lett. 129, 17–19 (1995).
Sugiyama, H. & Hayama, T. Abdominal viscera as site of emetic action for staphylococcal enterotoxin in the monkey. J. Infect. Dis. 115, 330–336 (1965).
Hu, D.-L. et al. Staphylococcal enterotoxin induces emesis through increasing serotonin release in intestine and it is downregulated by cannabinoid receptor 1. Cell. Microbiol. 9, 2267–2277 (2007).
Friedland, R. P. & Chapman, M. R. The role of microbial amyloid in neurodegeneration. PLoS Pathog. 13, e1006654 (2017).
Chapman, M. R. et al. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science 295, 851–855 (2002).
Collinson, S. K., Emödy, L., Müller, K. H., Trust, T. J. & Kay, W. W. Purification and characterization of thin, aggregative fimbriae from Salmonella enteritidis. J. Bacteriol. 173, 4773–4781 (1991).
Chen, S. G. et al. Exposure to the functional bacterial amyloid protein curli enhances α-synuclein aggregation in aged Fischer 344 rats and Caenorhabditis elegans. Sci. Rep. 6, 34477 (2016).
Russell, D. W. The enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem. 72, 137–174 (2003).
Hylemon, P. B. et al. Bile acids as regulatory molecules. J. Lipid Res. 50, 1509–1520 (2009).
Keitel, V. et al. The bile acid receptor TGR5 (Gpbar-1) acts as a neurosteroid receptor in brain. Glia 58, 1794–1805 (2010).
Yang, A. H., Ishii, I. & Chun, J. In vivo roles of lysophospholipid receptors revealed by gene targeting studies in mice. Biochim. Biophys. Acta 1582, 197–203 (2002).
Mertens, K. L., Kalsbeek, A., Soeters, M. R. & Eggink, H. M. Bile acid signaling pathways from the enterohepatic circulation to the central nervous system. Front. Neurosci. 11, 617 (2017).
Singh, J., Metrani, R., Shivanagoudra, S. R., Jayaprakasha, G. K. & Patil, B. S. Review on bile acids: effects of the gut microbiome, interactions with dietary fiber, and alterations in the bioaccessibility of bioactive compounds. J. Agric. Food Chem. 67, 9124–9138 (2019).
Philipp, B. Bacterial degradation of bile salts. Appl. Microbiol. Biotechnol. 89, 903–915 (2011).
Lund, E. G., Guileyardo, J. M. & Russell, D. W. cDNA cloning of cholesterol 24-hydroxylase, a mediator of cholesterol homeostasis in the brain. Proc. Natl Acad. Sci. USA 96, 7238–7243 (1999).
Kim, H. J. et al. Common CYP7A1 promoter polymorphism associated with risk of neuromyelitis optica. Neurobiol. Dis. 37, 349–355 (2010).
Båvner, A. et al. On the mechanism of accumulation of cholestanol in the brain of mice with a disruption of sterol 27-hydroxylase. J. Lipid Res. 51, 2722–2730 (2010).
Kotti, T. J., Ramirez, D. M. O., Pfeiffer, B. E., Huber, K. M. & Russell, D. W. Brain cholesterol turnover required for geranylgeraniol production and learning in mice. Proc. Natl Acad. Sci. USA 103, 3869–3874 (2006).
McMillin, M. & DeMorrow, S. Effects of bile acids on neurological function and disease. FASEB J. 30, 3658–3668 (2016).
McMillin, M. et al. TGR5 signaling reduces neuroinflammation during hepatic encephalopathy. J. Neurochem. 135, 565–576 (2015).
MacLennan, A. J. et al. An essential role for the H218/AGR16/Edg-5/LPB2 sphingosine 1-phosphate receptor in neuronal excitability. Eur. J. Neurosci. 14, 203–209 (2001).
Gohlke, H., Schmitz, B., Sommerfeld, A., Reinehr, R. & Häussinger, D. α5β1-Integrins are sensors for tauroursodeoxycholic acid in hepatocytes. Hepatology 57, 1117–1129 (2013).
Schubring, S. R., Fleischer, W., Lin, J. S., Haas, H. L. & Sergeeva, O. A. The bile steroid chenodeoxycholate is a potent antagonist at NMDA and GABAA receptors. Neurosci. Lett. 506, 322–326 (2012).
Yanovsky, Y. et al. Waking action of ursodeoxycholic acid (UDCA) involves histamine and GABAA receptor block. PLoS ONE 7, e42512 (2012).
MNeilly, A. D. et al. Bile acids modulate glucocorticoid metabolism and the hypothalamic–pituitary–adrenal axis in obstructive jaundice. J. Hepatol. 52, 705–711 (2010).
Quinn, M. et al. Suppression of the HPA axis during extrahepatic biliary obstruction induces cholangiocyte proliferation in the rat. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G182–G193 (2011).
Hertel, J. et al. Integrated analyses of microbiome and longitudinal metabolome data reveal microbial–host interactions on sulfur metabolism in Parkinson’s disease. Cell Rep. 29, 1767–1777.e8 (2019).
Nho, K. et al. Altered bile acid profile in mild cognitive impairment and Alzheimer’s disease: relationship to neuroimaging and CSF biomarkers. Alzheimers Dement. 15, 232–244 (2019).
MahmoudianDehkordi, S. et al. Altered bile acid profile associates with cognitive impairment in Alzheimer’s disease — an emerging role for gut microbiome. Alzheimers Dement. 15, 76–92 (2019).
Ho, P. P. & Steinman, L. Obeticholic acid, a synthetic bile acid agonist of the farnesoid X receptor, attenuates experimental autoimmune encephalomyelitis. Proc. Natl Acad. Sci. USA 113, 1600–1605 (2016).
Bhargava, P. et al. Bile acid metabolism is altered in multiple sclerosis and supplementation ameliorates neuroinflammation. J. Clin. Invest. 130, 3467–3482 (2020).
Wang, G. et al. Gut microbiota and relevant metabolites analysis in alcohol dependent mice. Front. Microbiol. 9, 1874 (2018).
Golubeva, A. V. et al. Microbiota-related changes in bile acid & tryptophan metabolism are associated with gastrointestinal dysfunction in a mouse model of autism. EBioMedicine 24, 166–178 (2017).
Swain, M. G., Patchev, V., Vergalla, J., Chrousos, G. & Jones, E. A. Suppression of hypothalamic–pituitary–adrenal axis responsiveness to stress in a rat model of acute cholestasis. J. Clin. Invest. 91, 1903–1908 (1993).
Rodrigues, C. M. P. et al. Neuroprotection by a bile acid in an acute stroke model in the rat. J. Cereb. Blood Flow. Metab. 22, 463–471 (2002).
Vaz, A. R. et al. Glycoursodeoxycholic acid reduces matrix metalloproteinase-9 and caspase-9 activation in a cellular model of superoxide dismutase-1 neurodegeneration. Mol. Neurobiol. 51, 864–877 (2015).
Chakrabarti, A. et al. Transcriptomics-driven lipidomics (TDL) identifies the microbiome-regulated targets of ileal lipid metabolism. NPJ Syst. Biol. Appl. 3, 33 (2017).
Ghazalpour, A., Cespedes, I., Bennett, B. J. & Allayee, H. Expanding role of gut microbiota in lipid metabolism. Curr. Opin. Lipidol. 27, 141–147 (2016).
Velagapudi, V. R. et al. The gut microbiota modulates host energy and lipid metabolism in mice. J. Lipid Res. 51, 1101–1112 (2010).
Lukovac, S. et al. Differential modulation by Akkermansia muciniphila and Faecalibacterium prausnitzii of host peripheral lipid metabolism and histone acetylation in mouse gut organoids. mBio 5, e01438–14 (2014).
Fu, J. et al. The gut microbiome contributes to a substantial proportion of the variation in blood lipids. Circ.Res. 117, 817–824 (2015).
An, D. et al. Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell 156, 123–133 (2014).
Lee, G., Hasan, M., Kwon, O.-S. & Jung, B. H. Identification of altered metabolic pathways during disease progression in EAE mice via metabolomics and lipidomics. Neuroscience 416, 74–87 (2019).
Yano, J. M. et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276 (2015).
Bean, L. A., Ianov, L. & Foster, T. C. Estrogen receptors, the hippocampus, and memory. Neuroscientist 20, 534–545 (2014).
Nguyen, T.-V. Developmental effects of androgens in the human brain. J. Neuroendocrinol. https://doi.org/10.1111/jne.12486 (2018).
Oliveira, G. A. & Oliveira, R. F. Androgen modulation of social decision-making mechanisms in the brain: an integrative and embodied perspective. Front. Neurosci. 8, 29 (2014).
Bollinger, J. L., Salinas, I., Fender, E., Sengelaub, D. R. & Wellman, C. L. Gonadal hormones differentially regulate sex-specific stress effects on glia in the medial prefrontal cortex. J. Neuroendocrinol. https://doi.org/10.1111/jne.12762 (2019).
Cheng, J. et al. Exposure of hyperandrogen during pregnancy causes depression- and anxiety-like behaviors, and reduced hippocampal neurogenesis in rat offspring. Front. Neurosci. 13, 436 (2019).
Nead, K. T. Androgens and depression: a review and update. Curr. Opin. Endocrinol. Diabetes Obes. 26, 175–179 (2019).
Diotel, N. et al. Steroid transport, local synthesis, and signaling within the brain: roles in neurogenesis, neuroprotection, and sexual behaviors. Front. Neurosci. 12, 84 (2018).
Zhu, B. T. & Conney, A. H. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis 19, 1–27 (1998).
Hellman, L. et al. The fate of hydrocortisone-4-C14 in man. J. Clin. Invest. 33, 1106–1115 (1954).
Bokkenheuser, V. D. & Winter, J. Biotransformation of steroid hormones by gut bacteria. Am. J. Clin. Nutr. 33, 2502–2506 (1980).
Groh, H., Schade, K. & Hörhold-Schubert, C. Steroid metabolism with intestinal microorganisms. J. Basic Microbiol. 33, 59–72 (1993).
García-Gómez, E., González-Pedrajo, B. & Camacho-Arroyo, I. Role of sex steroid hormones in bacterial–host interactions. BioMed Res. Internat. https://doi.org/10.1155/2013/928290 (2013).
Gloux, K. et al. A metagenomic β-glucuronidase uncovers a core adaptive function of the human intestinal microbiome. Proc. Natl Acad. Sci. USA 108, 4539–4546 (2011).
Dabek, M., McCrae, S. I., Stevens, V. J., Duncan, S. H. & Louis, P. Distribution of β-glucosidase and β-glucuronidase activity and of β-glucuronidase gene gus in human colonic bacteria. FEMS Microbiol. Ecol. 66, 487–495 (2008).
Beaud, D., Tailliez, P. & Anba-Mondoloni, J. Genetic characterization of the β-glucuronidase enzyme from a human intestinal bacterium, Ruminococcus gnavus. Microbiology 151, 2323–2330 (2005).
McIntosh, F. M. et al. Phylogenetic distribution of genes encoding β-glucuronidase activity in human colonic bacteria and the impact of diet on faecal glycosidase activities. Environ. Microbiol. 14, 1876–1887 (2012).
Ridlon, J. M. et al. Clostridium scindens: a human gut microbe with a high potential to convert glucocorticoids into androgens. J. Lipid Res. 54, 2437–2449 (2013).
Devendran, S., Mythen, S. M. & Ridlon, J. M. The desA and desB genes from Clostridium scindens ATCC 35704 encode steroid-17,20-desmolase. J. Lipid Res. 59, 1005–1014 (2018).
Winter, J. & Bokkenheuser, V. D. 21-Dehydroxylation of corticoids by anaerobic bacteria isolated from human fecal flora. J. Steroid Biochem. 9, 379–384 (1978).
Cerone-McLernon, A. M., Winter, J., Mosbach, E. H. & Bokkenheuser, V. D. Side-chain cleavage of cortisol by fecal flora. Biochim. Biophys. Acta Lipids Lipid Metab. 666, 341–347 (1981).
Ojanotko-Harri, A., Nikkari, T., Harrl, M.-P. & Paunio, K. Metabolism of progesterone and testosterone by Bacillus cereus strain Socransky 67 and Streptococcus mutans strain Ingbritt. Oral. Microbiol. Immunol. 5, 237–239 (1990).
Soory, M. Bacterial steroidogenesis by periodontal pathogens and the effect of bacterial enzymes on steroid conversions by human gingival fibroblasts in culture. J. Periodontal Res. 30, 124–131 (1995).
Lombardi, P., Goldin, B., Boutin, E. & Gorbach, S. L. Metabolism of androgens and estrogens by human fecal microorganisms. J. Steroid Biochem. 9, 795–801 (1978).
Järvenpää, P., Kosunen, T., Fotsis, T. & Adlercreutz, H. In vitro metabolism of estrogens by isolated intestinal micro-organisms and by human faecal microflora. J. Steroid Biochem. 13, 345–349 (1980).
Flores, R. et al. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J. Transl Med. 10, 253 (2012).
Plottel, C. S. & Blaser, M. J. Microbiome and malignancy. Cell Host Microbe 10, 324–335 (2011).
Fuhrman, B. J. et al. Associations of the fecal microbiome with urinary estrogens and estrogen metabolites in postmenopausal women. J. Clin. Endocrinol. Metab. 99, 4632–4640 (2014).
Goedert, J. J. et al. Investigation of the association between the fecal microbiota and breast cancer in postmenopausal women: a population-based case-control pilot study. J. Natl Cancer Inst. https://doi.org/10.1093/jnci/djv147 (2015).
Villa, A., Vegeto, E., Poletti, A. & Maggi, A. Estrogens, neuroinflammation, and neurodegeneration. Endocr. Rev. 37, 372–402 (2016).
Baker, J. M., Al-Nakkash, L. & Herbst-Kralovetz, M. M. Estrogen–gut microbiome axis: physiological and clinical implications. Maturitas 103, 45–53 (2017).
Kaliannan, K. et al. Estrogen-mediated gut microbiome alterations influence sexual dimorphism in metabolic syndrome in mice. Microbiome 6, 25 (2018).
Markle, J. G. M. et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339, 1084–1088 (2013).
Senghor, B., Sokhna, C., Ruimy, R. & Lagier, J.-C. Gut microbiota diversity according to dietary habits and geographical provenance. Hum. Microbiome J. 7–8, 1–9 (2018).
Sasabe, J. et al. Interplay between microbial D-amino acids and host D-amino acid oxidase modifies murine mucosal defence and gut microbiota. Nat. Microbiol. 1, 16125 (2016).
Metges, C. C. Contribution of microbial amino acids to amino acid homeostasis of the host. J. Nutr. 130, 1857S–1864S (2000).
Dodd, D. et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 551, 648–652 (2017).
Wikoff, W. R. et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. PNAS 106, 3698–3703 (2009).
Asano, Y. et al. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am. J. Physiol. Gastrointest. Liver Physiol. 303, G1288–G1295 (2012).
Tsavkelova, E. A., Botvinko, I. V., Kudrin, V. S. & Oleskin, A. V. Detection of neurotransmitter amines in microorganisms with the use of high-performance liquid chromatography. Dokl. Biochem. 372, 115–117 (2000).
Sperandio, V., Torres, A. G., Jarvis, B., Nataro, J. P. & Kaper, J. B. Bacteria–host communication: the language of hormones. PNAS 100, 8951–8956 (2003).
Kiraly, D. D. et al. Alterations of the host microbiome affect behavioral responses to cocaine. Sci. Rep. 6, 35455 (2016).
O’Farrell, K. & Harkin, A. Stress-related regulation of the kynurenine pathway: relevance to neuropsychiatric and degenerative disorders. Neuropharmacology 112, 307–323 (2017).
Jaglin, M. et al. Indole, a signaling molecule produced by the gut microbiota, negatively impacts emotional behaviors in rats. Front. Neurosci. 12, 216 (2018).
Zucchi, R., Chiellini, G., Scanlan, T. S. & Grandy, D. K. Trace amine-associated receptors and their ligands. Br. J. Pharmacol. 149, 967–978 (2006).
Schwarcz, R., Bruno, J. P., Muchowski, P. J. & Wu, H.-Q. Kynurenines in the mammalian brain: when physiology meets pathology. Nat. Rev. Neurosci. 13, 465–477 (2012).
Yanovsky, I. et al. Carbamate derivatives of indolines as cholinesterase inhibitors and antioxidants for the treatment of Alzheimer’s disease. J. Med. Chem. 55, 10700–10715 (2012).
Adesso, S. et al. Indoxyl sulfate affects glial function increasing oxidative stress and neuroinflammation in chronic kidney disease: interaction between astrocytes and microglia. Front. Pharmacol. 8, 370 (2017).
Needham, B. D. et al. Plasma and fecal metabolite profiles in autism spectrum disorder. bioRxiv https://doi.org/10.1101/2020.05.17.098806 (2020).
Gabriele, S. et al. Urinary p-cresol is elevated in young French children with autism spectrum disorder: a replication study. Biomarkers 19, 463–470 (2014).
Gacias, M. et al. Microbiota-driven transcriptional changes in prefrontal cortex override genetic differences in social behavior. eLife 5, e13442 (2016).
Zhu, L. et al. Structure and regulation of the gab gene cluster, involved in the γ-aminobutyric acid shunt, are controlled by a σ54 factor in Bacillus thuringiensis. J. Bacteriol. 192, 346–355 (2010).
O’Byrne, C. P. & Karatzas, K. A. G. The role of sigma B (σB) in the stress adaptations of Listeria monocytogenes: overlaps between stress adaptation and virulence. Adv. Appl. Microbiol. 65, 115–140 (2008).
Olson, C. A. et al. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell 173, 1728–1741 (2018).
Pokusaeva, K. et al. GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neurogastroenterol. Motil. 29, e12904 (2017).
Strandwitz, P. et al. GABA-modulating bacteria of the human gut microbiota. Nat. Microbiol. 4, 396–403 (2019).
Horder, J. et al. Glutamate and GABA in autism spectrum disorder — a translational magnetic resonance spectroscopy study in man and rodent models. Transl Psychiatry 8, 106 (2018).
Femenía, T., Gómez-Galán, M., Lindskog, M. & Magara, S. Dysfunctional hippocampal activity affects emotion and cognition in mood disorders. Brain Res. 1476, 58–70 (2012).
Soeiro-de-Souza, M. G. et al. Anterior cingulate glutamate–glutamine cycle metabolites are altered in euthymic bipolar I disorder. Eur. Neuropsychopharmacol. 25, 2221–2229 (2015).
Cherlyn, S. Y. T. et al. Genetic association studies of glutamate, GABA and related genes in schizophrenia and bipolar disorder: a decade of advance. Neurosci. Biobehav. Rev. 34, 958–977 (2010).
Williams, K., Zappia, A. M., Pritchett, D. B., Shen, Y. M. & Molinoff, P. B. Sensitivity of the N-methyl-D-aspartate receptor to polyamines is controlled by NR2 subunits. Mol. Pharmacol. 45, 803–809 (1994).
Matsumoto, M. et al. Impact of intestinal microbiota on intestinal luminal metabolome. Sci. Rep. 2, 233 (2012).
Sugiyama, Y. et al. Analysis of polyamine biosynthetic- and transport ability of human indigenous Bifidobacterium. Biosci. Biotechnol. Biochem. 82, 1606–1614 (2018).
Akasaka, N. & Fujiwara, S. The therapeutic and nutraceutical potential of agmatine, and its enhanced production using Aspergillus oryzae. Amino Acids https://doi.org/10.1007/s00726-019-02720-7 (2019).
Barua, S., Kim, J. Y., Kim, J. Y., Kim, J. H. & Lee, J. E. Therapeutic effect of agmatine on neurological disease: focus on ion channels and receptors. Neurochem. Res. 44, 735–750 (2019).
Deka, G., Bharath, S. R., Savithri, H. S. & Murthy, M. R. N. Structural studies on the decameric S. typhimurium arginine decarboxylase (ADC): pyridoxal 5′-phosphate binding induces conformational changes. Biochem. Biophys. Res. Commun. 490, 1362–1368 (2017).
Andréll, J. et al. Crystal structure of the acid-induced arginine decarboxylase from Escherichia coli: reversible decamer assembly controls enzyme activity. Biochemistry 48, 3915–3927 (2009).
Wu, N., Su, R.-B. & Li, J. Agmatine and imidazoline receptors: their role in opioid analgesia, tolerance and dependence. Cell Mol. Neurobiol. 28, 629–641 (2008).
Taksande, B. G. et al. Agmatine, an endogenous imidazoline receptor ligand modulates ethanol anxiolysis and withdrawal anxiety in rats. Eur. J. Pharmacol. 637, 89–101 (2010).
Sameer, S., Chakraborty, S. & Ugale, R. Agmatine attenuates acquisition but not the expression of ethanol conditioned place preference in mice: a role for imidazoline receptors. Behav. Pharmacol. 24, 87–94 (2013).
Shopsin, B. The clinical antidepressant effect of exogenous agmatine is not reversed by parachlorophenylalanine: a pilot study. Acta Neuropsychiatr. 25, 113–118 (2013).
Gupta, V. K. et al. Restoring polyamines protects from age-induced memory impairment in an autophagy-dependent manner. Nat. Neurosci. 16, 1453–1460 (2013).
Kang, S. et al. Agmatine ameliorates type 2 diabetes induced-Alzheimer’s disease-like alterations in high-fat diet-fed mice via reactivation of blunted insulin signalling. Neuropharmacology 113, 467–479 (2017).
Li, J., Doyle, K. M. & Tatlisumak, T. Polyamines in the brain: distribution, biological interactions, and their potential therapeutic role in brain ischaemia. Curr. Med. Chem. 14, 1807–1813 (2007).
Lyte, M. Microbial endocrinology: host–microbiota neuroendocrine interactions influencing brain and behavior. Gut Microbes 5, 381–389 (2014).
Koh, A., De Vadder, F., Kovatcheva-Datchary, P. & Bäckhed, F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165, 1332–1345 (2016).
Macfarlane, G. T. & Macfarlane, S. Bacteria, colonic fermentation, and gastrointestinal health. J. AOAC Int. 40, 50–60 (2019).
Sadler, R. et al. Short-chain fatty acids improve poststroke recovery via immunological mechanisms. J. Neurosci. 40, 1162–1173 (2020).
Frost, G. et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 5, 3611 (2014).
Wyss, M. T., Magistretti, P. J., Buck, A. & Weber, B. Labeled acetate as a marker of astrocytic metabolism. J. Cereb. Blood Flow. Metab. 31, 1668–1674 (2011).
Hoyles, L. et al. Microbiome–host systems interactions: protective effects of propionate upon the blood–brain barrier. Microbiome 6, 55 (2018).
Duscha, A. et al. Propionic acid shapes the multiple sclerosis disease course by an immunomodulatory mechanism. Cell 180, 1067–1080 (2020).
MacFabe, D. F. et al. A novel rodent model of autism: intraventricular infusions of propionic acid increase locomotor activity and induce neuroinflammation and oxidative stress in discrete regions of adult rat brain. Am. J. Biochem. Biotechnol. 4, 146–166 (2008).
Shultz, S. R. et al. Intracerebroventricular injections of the enteric bacterial metabolic product propionic acid impair cognition and sensorimotor ability in the Long–Evans rat: further development of a rodent model of autism. Behav. Brain Res. 200, 33–41 (2009).
Sleiman, S. F. et al. Putting the ‘HAT’ back on survival signalling: the promises and challenges of HDAC inhibition in the treatment of neurological conditions. Expert Opin. Investigat. Drugs 18, 573–584 (2009).
Govindarajan, N., Agis-Balboa, R. C., Walter, J., Sananbenesi, F. & Fischer, A. Sodium butyrate improves memory function in an Alzheimer’s disease mouse model when administered at an advanced stage of disease progression. J. Alzheimers Dis. 26, 187–197 (2011).
Kilgore, M. et al. Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology 35, 870–880 (2010).
da Silva, P. F. et al. Memory impairment induced by brain iron overload is accompanied by reduced H3K9 acetylation and ameliorated by sodium butyrate. Neuroscience 200, 42–49 (2012).
Dash, P. K., Orsi, S. A. & Moore, A. N. Histone deactylase inhibition combined with behavioral therapy enhances learning and memory following traumatic brain injury. Neuroscience 163, 1–8 (2009).
Steckert, A. V. et al. Effects of sodium butyrate on aversive memory in rats submitted to sepsis. Neurosci. Lett. 595, 134–138 (2015).
Barichello, T. et al. Sodium butyrate prevents memory impairment by re-establishing BDNF and GDNF expression in experimental pneumococcal meningitis. Mol. Neurobiol. 52, 734–740 (2015).
Kim, H. J. & Chuang, D.-M. HDAC inhibitors mitigate ischemia-induced oligodendrocyte damage: potential roles of oligodendrogenesis, VEGF, and anti-inflammation. Am. J. Transl Res. 6, 206–223 (2014).
Gardian, G. et al. Neuroprotective effects of phenylbutyrate in the N171-82Q transgenic mouse model of Huntington’s disease. J. Biol. Chem. 280, 556–563 (2005).
Ferrante, R. J. et al. Histone deacetylase inhibition by sodium butyrate chemotherapy ameliorates the neurodegenerative phenotype in Huntington’s disease mice. J. Neurosci. 23, 9418–9427 (2003).
Kidd, S. K. & Schneider, J. S. Protection of dopaminergic cells from MPP+-mediated toxicity by histone deacetylase inhibition. Brain Res. 1354, 172–178 (2010).
Crozier, A., Clifford, M. N. & Ashihara, H. Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet (Wiley, 2008).
Manach, C., Williamson, G., Morand, C., Scalbert, A. & Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 81, 230S–242S (2005).
Sadeghi Ekbatan, S. et al. Absorption and metabolism of phenolics from digests of polyphenol-rich potato extracts using the Caco-2/HepG2 co-culture system. Foods 7, 8 (2018).
Marín, L., Miguélez, E. M., Villar, C. J. & Lombó, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: antimicrobial properties. Biomed. Res. Int. https://doi.org/10.1155/2015/905215 (2015).
Liu, Z. & Hu, M. Natural polyphenol disposition via coupled metabolic pathways. Expert Opin. Drug Metab. Toxicol. 3, 389–406 (2007).
Ferruzzi, M. G. et al. Bioavailability of gallic acid and catechins from grape seed polyphenol extract is improved by repeated dosing in rats: implications for treatment in Alzheimer’s disease. J. Alzheimers Dis. 18, 113–124 (2009).
Ho, L. et al. Identification of brain-targeted bioactive dietary quercetin-3-O-glucuronide as a novel intervention for Alzheimer’s disease. FASEB J. 27, 769–781 (2013).
Wang, J. et al. Brain-targeted proanthocyanidin metabolites for Alzheimer’s disease treatment. J. Neurosci. 32, 5144–5150 (2012).
Wang, D. et al. Role of intestinal microbiota in the generation of polyphenol-derived phenolic acid mediated attenuation of Alzheimer’s disease β-amyloid oligomerization. Mol. Nutr. Food Res. 59, 1025–1040 (2015).
Loureiro, J. A. et al. Resveratrol and grape extract-loaded solid lipid nanoparticles for the treatment of Alzheimer’s disease. Molecules 22, 277 (2017).
Wang, J. et al. Targeting multiple pathogenic mechanisms with polyphenols for the treatment of Alzheimer’s disease — experimental approach and therapeutic implications. Front. Aging Neurosci. 6, 42 (2014).
Wang, J. et al. Epigenetic modulation of inflammation and synaptic plasticity promotes resilience against stress in mice. Nat. Commun. 9, 477 (2018).
Tomaro-Duchesneau, C. et al. Probiotic ferulic acid esterase active Lactobacillus fermentum NCIMB 5221 APA microcapsules for oral delivery: preparation and in vitro characterization. Pharmaceuticals 5, 236–248 (2012).
Ren, Z. et al. Ferulic acid exerts neuroprotective effects against cerebral ischemia/reperfusion-induced injury via antioxidant and anti-apoptotic mechanisms in vitro and in vivo. Int. J. Mol. Med. 40, 1444–1456 (2017).
Mori, T., Koyama, N., Guillot-Sestier, M.-V., Tan, J. & Town, T. Ferulic acid is a nutraceutical β-secretase modulator that improves behavioral impairment and Alzheimer-like pathology in transgenic mice. PLoS ONE 8, e55774 (2013).
Zeni, A. L. B., Camargo, A. & Dalmagro, A. P. Ferulic acid reverses depression-like behavior and oxidative stress induced by chronic corticosterone treatment in mice. Steroids 125, 131–136 (2017).
Wang, J. et al. Cocoa extracts reduce oligomerization of amyloid-β: implications for cognitive improvement in Alzheimer’s disease. J. Alzheimers Dis. 41, 643–650 (2014).
Santa-Maria, I. et al. GSPE interferes with tau aggregation in vivo: implication for treating tauopathy. Neurobiol. Aging 33, 2072–2081 (2012).
Bode, L. M. et al. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am. J. Clin. Nutr. 97, 295–309 (2013).
Zhang, L.-F. et al. Resveratrol alleviates motor and cognitive deficits and neuropathology in the A53T α-synuclein mouse model of Parkinson’s disease. Food Funct. 9, 6414–6426 (2018).
Sampson, T. R. et al. A gut bacterial amyloid promotes α-synuclein aggregation and motor impairment in mice. eLife 9, e53111 (2020).
Clavel, T., Borrmann, D., Braune, A., Doré, J. & Blaut, M. Occurrence and activity of human intestinal bacteria involved in the conversion of dietary lignans. Anaerobe 12, 140–147 (2006).
Rafii, F. The role of colonic bacteria in the metabolism of the natural isoflavone daidzin to equol. Metabolites 5, 56–73 (2015).
Rietjens, I. M. C. M., Louisse, J. & Beekmann, K. The potential health effects of dietary phytoestrogens. Br. J. Pharmacol. 174, 1263–1280 (2017).
Sakai, T. & Kogiso, M. Soy isoflavones and immunity. J. Med. Invest. 55, 167–173 (2008).
Mueller, S. O., Simon, S., Chae, K., Metzler, M. & Korach, K. S. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor alpha (ERα) and ERβ in human cells. Toxicol. Sci. 80, 14–25 (2004).
Cooke, P. S., Selvaraj, V. & Yellayi, S. Genistein, estrogen receptors, and the acquired immune response. J. Nutr. 136, 704–708 (2006).
Little, M. S., Pellock, S. J., Walton, W. G., Tripathy, A. & Redinbo, M. R. Structural basis for the regulation of β-glucuronidase expression by human gut Enterobacteriaceae. Proc. Natl Acad. Sci. USA 115, E152–E161 (2018).
Roberts, M. S., Magnusson, B. M., Burczynski, F. J. & Weiss, M. Enterohepatic circulation. Clin. Pharmacokinet. 41, 751–790 (2002).
Krishnaswamy, S. et al. Serotonin (5-hydroxytryptamine) glucuronidation in vitro: assay development, human liver microsome activities and species differences. Xenobiotica 33, 169–180 (2003).
Guthrie, L., Wolfson, S. & Kelly, L. The human gut chemical landscape predicts microbe-mediated biotransformation of foods and drugs. eLife 8, e42866 (2019).
Winter, J. & Bokkenheuser, V. D. Bacterial metabolism of natural and synthetic sex hormones undergoing enterohepatic circulation. J. Steroid Biochem. 27, 1145–1149 (1987).
Magnúsdóttir, S., Ravcheev, D., de Crécy-Lagard, V. & Thiele, I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front. Genet. 6, 148 (2015).
Rowland, I. et al. Gut microbiota functions: metabolism of nutrients and other food components. Eur. J. Nutr. 57, 1–24 (2018).
LeBlanc, J. G. et al. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr. Opin. Biotechnol. 24, 160–168 (2013).
Hiratsuka, T. et al. An alternative menaquinone biosynthetic pathway operating in microorganisms. Science 321, 1670–1673 (2008).
Ferland, G. Vitamin K and brain function. Semin. Thromb. Hemost. 39, 849–855 (2013).
Derrien, M. et al. Modulation of mucosal immune response, tolerance, and proliferation in mice colonized by the mucin-degrader Akkermansia muciniphila. Front. Microbiol. 2, 166 (2011).
Yang, S., Minkler, P. & Hoppel, C. cis-3,4-Methylene-heptanoylcarnitine: characterization and verification of the C8:1 acylcarnitine in human urine. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 857, 251–258 (2007).
Zhang, L. S. & Davies, S. S. Microbial metabolism of dietary components to bioactive metabolites: opportunities for new therapeutic interventions. Genome Med. 8, 46 (2016).
Ogawa, J. et al. Production of conjugated fatty acids by lactic acid bacteria. J. Biosci. Bioeng. 100, 355–364 (2005).
Sberro, H. et al. Large-scale analyses of human microbiomes reveal thousands of small, novel genes. Cell 178, 1245–1259.e14 (2019).
Bercik, P. et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut–brain communication. Neurogastroenterol. Motil. 23, 1132–1139 (2011).
Sgritta, M. et al. Mechanisms underlying microbial-mediated changes in social behavior in mouse models of autism spectrum disorder. Neuron 101, 246–259.e6 (2019).
Goehler, L. E. et al. Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain Behav. Immun. 19, 334–344 (2005).
Gershon, M. D. & Tack, J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 132, 397–414 (2007).
Vadder, F. D. et al. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc. Natl Acad. Sci. USA 115, 6458–6463 (2018).
Sjögren, K. et al. The gut microbiota regulates bone mass in mice. J. Bone Miner. Res. 27, 1357–1367 (2012).
Tian, P., Wang, G., Zhao, J., Zhang, H. & Chen, W. Bifidobacterium with the role of 5-hydroxytryptophan synthesis regulation alleviates the symptom of depression and related microbiota dysbiosis. J. Nutr. Biochem. 66, 43–51 (2019).
Lund, M. L. et al. Enterochromaffin 5-HT cells — a major target for GLP-1 and gut microbial metabolites. Mol. Metab. 11, 70–83 (2018).
Wang, H. et al. TLR2 plays a pivotal role in mediating mucosal serotonin production in the gut. J. Immunol. 202, 3041–3052 (2019).
Kidd, M. et al. Luminal regulation of normal and neoplastic human EC cell serotonin release is mediated by bile salts, amines, tastants, and olfactants. Am. J. Physiol. Gastrointest. Liver Physiol. 295, G260–G272 (2008).
Tsuruta, T. et al. Organoids as an ex vivo model for studying the serotonin system in the murine small intestine and colon epithelium. Biochem. Biophys. Res. Commun. 474, 161–167 (2016).
Reigstad, C. S. et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 29, 1395–1403 (2015).
Neufeld, K.-A. M. et al. Oral selective serotonin reuptake inhibitors activate vagus nerve dependent gut–brain signalling. Sci. Rep. 9, 1–11 (2019).
Ma, Q. et al. Impact of microbiota on central nervous system and neurological diseases: the gut–brain axis. J. Neuroinflammation 16, 53 (2019).
Frenois, F. et al. Lipopolysaccharide induces delayed FosB/ΔFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology 32, 516–531 (2007).
Macfarlane, S., Cleary, S., Bahrami, B., Reynolds, N. & Macfarlane, G. T. Synbiotic consumption changes the metabolism and composition of the gut microbiota in older people and modifies inflammatory processes: a randomised, double-blind, placebo-controlled crossover study. Aliment. Pharmacol. Ther. 38, 804–816 (2013).
Kuo, S.-M. The interplay between fiber and the intestinal microbiome in the inflammatory response. Adv. Nutr. 4, 16–28 (2013).
Lee, S. U. et al. β-Arrestin 2 mediates G protein-coupled receptor 43 signals to nuclear factor-κB. Biol. Pharm. Bull. 36, 1754–1759 (2013).
Patnala, R., Arumugam, T. V., Gupta, N. & Dheen, S. T. HDAC inhibitor sodium butyrate-mediated epigenetic regulation enhances neuroprotective function of microglia during ischemic stroke. Mol. Neurobiol. 54, 6391–6411 (2017).
Erny, D. et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 18, 965–977 (2015).
Borges, G., Lean, M. E. J., Roberts, S. A. & Crozier, A. Bioavailability of dietary (poly)phenols: a study with ileostomists to discriminate between absorption in small and large intestine. Food Funct. 4, 754–762 (2013).
Pimpão, R. C., Ventura, M. R., Ferreira, R. B., Williamson, G. & Santos, C. N. Phenolic sulfates as new and highly abundant metabolites in human plasma after ingestion of a mixed berry fruit purée. Br. J. Nutr. 113, 454–463 (2015).
Gasperotti, M. et al. Fate of microbial metabolites of dietary polyphenols in rats: is the brain their target destination? ACS Chem. Neurosci. 6, 1341–1352 (2015).
Chen, T.-Y. et al. Plasma bioavailability and regional brain distribution of polyphenols from apple/grape seed and bilberry extracts in a young swine model. Mol. Nutr. Food Res. 59, 2432–2447 (2015).
Figueira, I. et al. Polyphenols journey through blood–brain barrier towards neuronal protection. Sci. Rep. 7, 1–16 (2017).
Youdim, K. A. et al. Interaction between flavonoids and the blood–brain barrier: in vitro studies. J. Neurochem. 85, 180–192 (2003).
Zhao, W. et al. Novel application of brain-targeting polyphenol compounds in sleep deprivation-induced cognitive dysfunction. Neurochem. Int. 89, 191–197 (2015).
Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 1693, 128–133 (2018).
Cameron, J. S. et al. Toll-like receptor 3 is a potent negative regulator of axonal growth in mammals. J. Neurosci. 27, 13033–13041 (2007).
Ma, Y. et al. Toll-like receptor 8 functions as a negative regulator of neurite outgrowth and inducer of neuronal apoptosis. J. Cell Biol. 175, 209–215 (2006).
Okun, E. et al. TLR2 activation inhibits embryonic neural progenitor cell proliferation. J. Neurochem. 114, 462–474 (2010).
Janssens, Y. et al. Screening of quorum sensing peptides for biological effects in neuronal cells. Peptides 101, 150–156 (2018).
Rothhammer, V. et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 22, 586–597 (2016).
Rothhammer, V. et al. Microglial control of astrocytes in response to microbial metabolites. Nature 557, 724 (2018).
Liu, F., Horton-Sparks, K., Hull, V., Li, R. W. & Martínez-Cerdeño, V. The valproic acid rat model of autism presents with gut bacterial dysbiosis similar to that in human autism. Mol. Autism 9, 61 (2018).
Ho, M.-F. et al. Ketamine and ketamine metabolites as novel estrogen receptor ligands: induction of cytochrome P450 and AMPA glutamate receptor gene expression. Biochem. Pharmacol. 152, 279–292 (2018).
Yang, T. et al. Butyrate regulates inflammatory cytokine expression without affecting oxidative respiration in primary astrocytes from spontaneously hypertensive rats. Physiol. Rep. 6, e13732 (2018).
Xiang, Y. et al. Acetylpuerarin inhibits oxygen-glucose deprivation-induced neuroinflammation of rat primary astrocytes via the suppression of HIF-1 signaling. Exp. Ther. Med. 16, 2689–2695 (2018).
Xiang, Y. et al. Anti-inflammatory effect of acetylpuerarin on eicosanoid signaling pathway in primary rat astrocytes. J. Mol. Neurosci. 52, 577–585 (2014).
Xin, Y. et al. Effects of oligosaccharides from Morinda officinalis on gut microbiota and metabolome of APP/PS1 transgenic mice. Front. Neurol. 9, 412 (2018).
Chen, H. et al. Gut microbiota interventions with Clostridium butyricum and norfloxacin modulate immune response in experimental autoimmune encephalomyelitis mice. Front. Immunol. 10, 1662 (2019).
Al-Ghezi, Z. Z., Busbee, P. B., Alghetaa, H., Nagarkatti, P. S. & Nagarkatti, M. Combination of cannabinoids, δ-9-tetrahydrocannabinol (THC) and cannabidiol (CBD), mitigates experimental autoimmune encephalomyelitis (EAE) by altering the gut microbiome. Brain Behav. Immun. https://doi.org/10.1016/j.bbi.2019.07.028 (2019).
Gandy, K. A. O., Zhang, J., Nagarkatti, P. & Nagarkatti, M. The role of gut microbiota in shaping the relapse–remitting and chronic–progressive forms of multiple sclerosis in mouse models. Sci. Rep. 9, 1–17 (2019).
Melbye, P., Olsson, A., Hansen, T. H., Søndergaard, H. B. & Oturai, A. B. Short-chain fatty acids and gut microbiota in multiple sclerosis. Acta Neurol. Scand. 139, 208–219 (2019).
Mangalam, A. et al. Human gut-derived commensal bacteria suppress CNS inflammatory and demyelinating disease. Cell Rep. 20, 1269–1277 (2017).
Poisson, L. M. et al. Untargeted plasma metabolomics identifies endogenous metabolite with drug-like properties in chronic animal model of multiple sclerosis. J. Biol. Chem. 290, 30697–30712 (2015).
Khalaj, A. J., Hasselmann, J., Augello, C., Moore, S. & Tiwari-Woodruff, S. K. Nudging oligodendrocyte intrinsic signaling to remyelinate and repair: estrogen receptor ligand effects. J. Steroid Biochem. Mol. Biol. 160, 43–52 (2016).
Khalaj, A. J. et al. Estrogen receptor (ER) β expression in oligodendrocytes is required for attenuation of clinical disease by an ERβ ligand. PNAS 110, 19125–19130 (2013).
Takao, T. et al. 17β-Estradiol protects oligodendrocytes from cytotoxicity induced cell death. J. Neurochem. 89, 660–673 (2004).
Voskuhl, R. R. et al. Gene expression in oligodendrocytes during remyelination reveals cholesterol homeostasis as a therapeutic target in multiple sclerosis. Proc. Natl Acad. Sci. USA 116, 10130–10139 (2019).
Rankin, K. A. et al. Selective estrogen receptor modulators enhance CNS remyelination independent of estrogen receptors. J. Neurosci. 39, 2184–2194 (2019).
Abbott, N. J., Rönnbäck, L. & Hansson, E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 7, 41–53 (2006).
Jin, L., Nation, R. L., Li, J. & Nicolazzo, J. A. Species-dependent blood–brain barrier disruption of lipopolysaccharide: amelioration by colistin in vitro and in vivo. Antimicrob. Agents Chemother. 57, 4336–4342 (2013).
Braniste, V. et al. The gut microbiota influences blood–brain barrier permeability in mice. Sci. Transl Med. 6, 263ra158 (2014).
Tang, A. T. et al. Endothelial TLR4 and the microbiome drive cerebral cavernous malformations. Nature 545, 305–310 (2017).
Wolf, S. A., Boddeke, H. W. G. M. & Kettenmann, H. Microglia in physiology and disease. Annu. Rev. Physiol. 79, 619–643 (2017).
Zhong, L.-M. et al. Resveratrol inhibits inflammatory responses via the mammalian target of rapamycin signaling pathway in cultured LPS-stimulated microglial cells. PLoS ONE 7, e32195 (2012).
Catorce, M. N. & Gevorkian, G. LPS-induced murine neuroinflammation model: main features and suitability for pre-clinical assessment of nutraceuticals. Curr. Neuropharmacol. 14, 155–164 (2016).
Yanguas-Casás, N., Barreda-Manso, M. A., Nieto-Sampedro, M. & Romero-Ramírez, L. TUDCA: an agonist of the bile acid receptor GPBAR1/TGR5 with anti-inflammatory effects in microglial cells. J. Cell. Physiol. 232, 2231–2245 (2017).
Author information
Authors and Affiliations
Contributions
S.K.M. and B.D.N. researched data for the article and made substantial contributions to the discussion of content, writing, reviewing and editing of the manuscript before submission. R.K.-D. contributed to the review and editing of the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
S.K.M. has financial interest in Axial Biotherapeutics. B.D.N. and R.K.-D. declare no competing interests.
Additional information
Peer review information
Nature Reviews Neuroscience thanks Peter Holzer, who co-reviewed with Aitak Farzi; John Cryan; and Mauro Costa-Mattioli for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Bile acids
-
Complex lipid products of host cholesterol metabolism that play a major role in fat digestion and signalling in energy metabolism. Host bile acids (primary bile acids) are commonly modified by bacteria into secondary bile acids.
- Enterochromaffin cells
-
Neuroendocrine cells in the gut lining that aid in gastrointestinal motility and produce 90% of the body’s serotonin in response to persistent intestinal signals.
- Germ-free mice
-
Mice reared in conditions completely absent of microbial exposure.
- Gut microbiota
-
An intestinal community comprising bacteria and other microorganisms including viruses, fungi, protists and archaea that permanently or transiently inhabit the lower gastrointestinal tract, especially the small intestine and colon.
- Microorganism-associated molecular patterns
-
(MAMPs). Well-conserved components of microbial cells that are acutely detected by the innate immune system of the host throughout the body, including the brain.
- Polyphenols
-
A vast class of thousands of plant-derived molecules containing at least one phenol group that are generally poorly absorbed by the host until being transformed by the gut microbiota into bioavailable and bioactive metabolites.
- Short-chain fatty acids
-
(SCFAs). Fatty acids with chains of fewer than six carbons that are the end product of bacterial fermentation of complex polysaccharides and serve as energy source and signalling molecule in the host.
- Specific pathogen-free mice
-
Mice conventionally colonized with a complete gut microbiota.
- Steroid hormones
-
Circulating signalling molecules derived from cholesterol with an organic chemical structure consisting of four carbon rings and various regulatory roles in the host.
- Vagus nerve
-
A principle neuronal connection between the gut and brain, comprising a bundle of neurons that sends and receives signals directly between gut tissue (and other organs) and the brainstem. These signals can then be further transmitted throughout the brain.
Rights and permissions
About this article
Cite this article
Needham, B.D., Kaddurah-Daouk, R. & Mazmanian, S.K. Gut microbial molecules in behavioural and neurodegenerative conditions. Nat Rev Neurosci 21, 717–731 (2020). https://doi.org/10.1038/s41583-020-00381-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41583-020-00381-0
This article is cited by
-
Pediococcus acidilactici reduces tau pathology and ameliorates behavioral deficits in models of neurodegenerative disorders
Cell Communication and Signaling (2024)
-
Microbiota-derived acetate attenuates neuroinflammation in rostral ventrolateral medulla of spontaneously hypertensive rats
Journal of Neuroinflammation (2024)
-
Immunological aspects of central neurodegeneration
Cell Discovery (2024)
-
Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases
Signal Transduction and Targeted Therapy (2024)
-
Gut-Brain Axis Deregulation and Its Possible Contribution to Neurodegenerative Disorders
Neurotoxicity Research (2024)