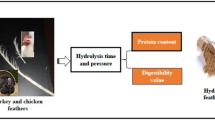
Abstract
The vast amount of feathers generated (>1 Mtons/a in Europe) in the poultry industry is an opportunity of upcycling by-product materials and improving sustainable practices. Feathers are potentially interesting materials as feed protein ingredients due to their high protein (>85 wt%) and cystine content (>7 wt%). However, due to their challenging recalcitrant nature, they have to be processed to make feather protein suitably digestible. The objective was to investigate the effects of temperature (120oC–160oC) and time (10, 30, and 60 min) in thermal pressure hydrolysis of feathers on availability for enzymatic hydrolysis (AEH) and cystine conservation. AEH is defined as degree of degradation of processed feather protein by two digestive enzymes pepsin and pancreatin (Boisen). The present study identified and assessed four temperature stages that take place during feather processing. The four temperature stages are 120oC–135oC, 140oC–155oC, > 160oC, and the cooling-down phase. The second stage has the greatest influence on AEH. As well as temperature, hydrolysis time is also an essential parameter that had a major impact in the second stage (140oC–155oC). Both temperature and time influence negatively cystine content and stability. The present study demonstrates for the first time the importance of four reaction stages during feather hydrolysis and the impact of four stages on AEH of the obtained products.
Similar content being viewed by others
Abbreviations
- AEH:
-
availability for enzymatic hydrolysis
- TPH:
-
thermal pressure hydrolysis; wt% CP, wt% of crude protein
- DSC:
-
Differential Scanning Calorimetry; µmol / 100 g CP, µmol / 100 g crude protein
References
Marquer, P., T. Rabade, and R. Forti (2015) Meat production statistics. https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Archive:Meat_production_statistics.
Food and Agriculture Organization of the United Nations (2017) Food outlook biannual report on global food markets. http://www.fao.org/giews/.
Onifade, A. A., N. A. Al-Sane, A. A. Al-Musallam, and S. Al-Zarban (1998) A review: Potentials for biotechnological applications of keratin-degrading microorganisms and their enzymes for nutritional improvement of feathers and other keratins as livestock feed resources. Bioresour. Technol. 66: 1–11.
Bureau, D. P. (2013) The nutritive value of processed animal proteins to different aquaculture species. Proceedings of EFPRA Congress 2013. June 12–15. Prague, Czech.
Meeker, D. L. and C. R. Hamilton (2006) An overview of the rendering industry. pp. 1–16. In: D. L. Meeker (ed.). Essential Rendering- All About the Animal By-products Industry. National Renderers Association, Alexandria, VA, USA.
Sevier, C. S. and C. A. Kaiser (2002) Formation and transfer of disulphide bonds in living cells. Nat. Rev. Mol. Cell Biol. 3: 836–847.
Yin, J., W. Ren, G. Yang, J. Duan, X. Huang, R. Fang, C. Li, T. Li, Y. Yin, Y. Hou, S. W. Kim, and G. Wu (2016) L-Cysteine metabolism and its nutritional implications. Mol. Nutr. Food Res. 60: 134–146.
Miller, E. L. (2002) Protein nutrition requirements of farmed livestock and dietary supply. Proceedings of FAO Expert Consultation and Workshop on Protein Sources for the Animal Feed Industry. April 29–May 3. Bangkok, Thailand.
Klemesrud, M. and T. J. Klopfenstein (1997) Cysteine from feather meal and sulfur amino acid requirements for growing steers. Neb. Beef Cattle Rep. 411: 14–15.
Woodgate, S. L. (2006) What would a world without rendering look like? pp. 277–294. In: D. L. Meeker (ed.). Essential Rendering- All About the Animal By-products Industry. National Renderers Association, Alexandria, VA, USA.
Wang, B., W. Yang, J. McKittrick, and M. A. Meyers (2016) Keratin: Structure, mechanical properties, occurrence in biological organisms, and efforts at bioinspiration. Prog. Mater. Sci. 76: 229–318.
Fraser, R. D. B., T. P. Macrae, D. A. D. Parry, and E. Suzuki (1969) The structure of β-keratin. Polymer. 10: 810–826.
Fraser, R. D. B. and D. A. D. Parry (2014) Amino acid sequence homologies in the hard keratins of birds and reptiles, and their implications for molecular structure and physical properties. J. Struct. Biol. 188: 213–224.
Barone, J. R., W. F. Schmidt, and N. T. Gregoire (2005) Extrusion of feather keratin. J. Appl. Polym. Sci. 100: 1432–1442.
Koenig, N. H. and M. Friedman (1977) Comparison of wool reactions with selected mono- and bifunctional reagents. pp. 355–382. In: M. Friedman (ed.). Protein Crosslinking. Plenum Press, New York, NY, USA.
Moran, E. T., J. D. Summers, and S. J. Slinger (1966) Keratin as a source of protein for the growing chick: 1. Amino acid imbalance as the cause for inferior performance of feather meal and the implication of disulfide bonding in raw feathers as the reason for poor digestibility. Poult. Sci. 45: 1257–1266.
Papadopoulos, M. C. (1984) Feather Meal: Evaluation of the Effect of Processing Conditions by Chemical and Chick Assays. Ph.D. Thesis. Wageningen University & Research, Wageningen, Netherlands.
Papadopoulos, M. C. (1986) The effect of enzymatic treatment on amino acid content and nitrogen characteristics of feather meal. Anim. Feed Sci. Technol. 16: 151–156.
Latshaw, J. D. (1990) Quality of feather meal as affected by feather processing conditions. Poult. Sci. 69: 953–958.
Coward-Kelly, G., V. S. Chang, F. K. Agbogbo, and M. T. Holtzapple (2006) Lime treatment of keratinous materials for the generation of highly digestible animal feed: 1. Chicken feathers. Bioresour. Technol. 97: 1337–1343.
Chen, J., S. Ding, Y. Ji, J. Ding, X. Yang, M. Zou, and Z. Li (2015) Microwave-enhanced hydrolysis of poultry feather to produce amino acid. Chem. Eng. Process. 87: 104–109.
Tadtiyanant, C., J. J. Lyons, and J. M. Vandepopulire (1993) Extrusion processing used to convert dead poultry, feathers, eggshells, hatchery waste, and mechanically deboned residue into feedstuffs for poultry. Poult. Sci. 72: 1515–1527.
Papadopoulos, M. C. (1989) Effect of processing on high-protein feedstuffs: A review. Biol Wastes. 29: 123–138.
Friedman, M. (1999) Chemistry, biochemistry, nutrition, and microbiology of lysinoalanine, lanthionine, and histidinoalanine in food and other proteins. J. Agric. Food Chem. 47: 1295–1319.
Commission regulation (EC) No 152/2009 2014 Determination of the content of crude ash, in No 152/2009. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02009R0152-20170524&from=EN.
Boisen, S. and B. O. Eggum (1991) Critical evaluation of in vitro methods for estimating digestibility in simple-stomach animals. Nutr Res Rev. 4: 141–162.
Boisen, S. and J. A. Fernhndez (1995) Prediction of the apparent ileal digestibility of protein and amino acids in feedstuffs and feed mixtures for pigs by in vitro analyses. Anim. Feed Sci. Technol. 51: 29–43.
ISO, Animal feeding stuffs — Determination of amino acids content.https://www.iso.org/standard/37258.html.
Milczarek, P., M. Zielinski, and M. L. Garcia (1992) The mechanism and stability of thermal transitions in hair keratin. Colloid Polym. Sci. 270: 1106–1115.
Takahashi, K., H. Yamamoto, Y. Yokote, and M. Hattori (2004) Thermal behavior of fowl feather keratin. Biosci. Biotechnol. Biochem. 68: 1875–1881.
Brebu, M. and I. Spiridon (2011) Thermal degradation of keratin waste. J. Anal. Appl. Pyrolysis. 91: 288–295.
Sohn, M. and C. T. Ho (1995) Ammonia generation during thermal degradation of amino acids. J. Agric. Food Chem. 43: 3001–3003.
Yablokov, V. A., Y. A. Vasina, I. A. Zelyaev, and S. V. Mitrofanova (2009) Kinetics of thermal decomposition of sulfur-containing amino acids. Russ. J. Gen. Chem. 79: 1141.
Lagrain, B., K. De Vleeschouwer, I. Rombouts, K. Brijs, M. E. Hendrickx, and J. A. Delcour (2010) The kinetics of β-elimination of cystine and the formation of lanthionine in gliadin. J. Agric. Food Chem. 58: 10761–10767.
Papadopoulos, M. C., A. R. El Boushy, A. E. Roodeen, and E. H. Ketelaars (1986) Effects of processing time and moisture content on amino acid composition and nitrogen characteristics of feather meal. Anim. Feed Sci. Technol. 14: 279–290.
Volkin, D. B. and A. M. Klibanov (1987) Thermal destruction processes in proteins involving cystine residues. J. Biol. Chem. 262: 2945–2950.
Papadopoulos, M. C., A. R. el Boushy, and E. H. Ketelaars (1985) Effect of different processing conditions on amino acid digestibility of feather meal determined by chicken assay. Poult. Sci. 64: 1729–1741.
Moritz, J. S. and J. D. Latshaw (2001) Indicators of nutritional value of hydrolyzed feather meal. Poult. Sci. 80: 79–86.
Asquith, R. S. and M. S. Otterburn (1977) Cystine-alkali reactions in relation to protein crosslinking. pp. 93–121. In: M. Friedman (ed.). Protein Crosslinking. Plenum Press, New York, NY, USA.
Singh, R. and G. M. Whitesides (1990) Comparisons of rate constants for thiolate-disulfide interchange in water and in polar aprotic solvents using dynamic proton NMR line shape analysis. J. Am. Chem. Soc. 112: 1190–1197.
Damodaran, S. (2007) Amino acids, peptides, and proteins. pp. 217–329. In: S. Domodaran, K. L. Parkin, and O. R Fennema (eds.). Fennema’s Food Chemistry. CRC Press, Bosa Roca, FL, USA.
Weder, J. K. P. and H. D. Belitz (2003) Interactions and reactions involved in food processing. pp. 4841–4847. In: B. Caballero, P. Finglas, and F. Toldra (eds.). Encyclopedia of Food Sciences and Nutrition. Academic Press, Cambridge, MA, USA.
Han, Y. and C. M. Parsons (1991) Protein and amino acid quality of feather meals. Poult. Sci. 70: 812–822.
Papadopoulos, M. C. (1985) Processed chicken feathers as feedstuff for poultry and swine. A review. Agric Wastes. 14: 275–290.
Latshaw, J. D., N. Musharaf, and R. Retrum (1994) Processing of feather meal to maximize its nutritional value for poultry. Anim. Feed Sci. Technol. 47: 179–188.
Sullivan, T. W. and E. L. Stephenson (1957) Effect of processing methods on the utilization of hydrolyzed poultry feathers by growing chicks. Poult. Sci. 36: 361–365.
Papadopoulos, M. C. (1987) In vitro and in vivo estimation of protein quality of laboratory treated feather meal. Biol Wastes. 21: 143–148.
Papadopoulos, M. C., A. R. El-Boushy, and A. E. Roodbeen (1985) The effect of varying autoclaving conditions and added sodium hydroxide on amino acid content and nitrogen characteristics of feather meal. J. Sci. Food. Agric. 36: 1219–1226.
Wang, Z., T. Rejtar, Z. S. Zhou, and B. L. Karger (2010) Desulfurization of cysteine-containing peptides resulting from sample preparation for protein characterization by mass spectrometry. Rapid Commun. Mass Spectrom. 24: 267–275.
Perna, A. F., M. Zacchia, F. Trepiccione, and D. Ingrosso (2017) The sulfur metabolite lanthionine: evidence for a role as a novel uremic toxin. Toxins. 9: 26.
Acknowledgments
This work was financially supported by Saria International GmbH.
The authors declare no conflict of interest.
Neither ethical approval nor informed consent was required for this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material (ESM)
Rights and permissions
About this article
Cite this article
Goerner-Hu, X., Scott, E.L., Seeger, T. et al. Reaction Stages of Feather Hydrolysis: Factors That Influence Availability for Enzymatic Hydrolysis and Cystine Conservation during Thermal Pressure Hydrolysis. Biotechnol Bioproc E 25, 749–757 (2020). https://doi.org/10.1007/s12257-019-0351-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-019-0351-8