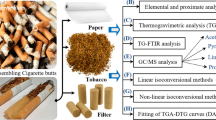
Abstract
The present study attempts to explore the energy potential of smoked cigarette butts (SCB) through pyrolysis. For the first time, the pyrolysis characteristics, including the kinetic triplet and the thermodynamic parameters, were investigated using non-isothermal thermogravimetry. Firstly, three pseudo-components were successfully deconvoluted from the multiple-step pyrolysis of SCB using the asymmetrical Fraser-Suzuki function, which corresponds to the devolatilization reactions of retained organic volatile components (PS-1), unburned tobacco (PS-2), and cellulose acetate fibers (PS-3). Posteriorly, the isoconversional methods of Friedman, Flynn-Wall-Ozawa, Kissinger-Akahira-Sunose, and Starink were used to obtain the activation energy values, which were lower for PS-1 (from 101.87 to 108.77 kJ mol−1). The frequency factor values for SCB pyrolysis determined by the compensation effect method were 1.77 × 1012 min−1 for PS-1, 9.44 × 1016 min−1 for PS-2, and 9.62 × 1020 min−1 for PS-3. According to the master plot method, the three pseudo-components followed nth-order reaction models. An acceptable correspondence was observed between experimental and reconstructed pyrolysis behavior, proving the representativity and reliability of the obtained kinetic triplets. Both positive values of ΔH and ΔG suggest that the pyrolytic conversion of smoked cigarette butts into biofuels can be considered as a non-spontaneous conversion. These pyrolysis-related findings from SCB can be used to offer a good opportunity for its valorization as an energy commodity instead of a neglected solid residue.

Graphical abstract




Similar content being viewed by others
Abbreviations
- A (min−1):
-
frequency factor
- a (dimensionless):
-
compensation parameter
- b (dimensionless):
-
compensation parameter
- c (dimensionless):
-
contributed fraction in conversion
- DTG (wt% min−1):
-
differential thermogravimetric
- (dα/dt)av (min−1):
-
average of experimental values
- (dα/dt)dec (min−1):
-
values from deconvolution
- (dα/dt)exp (min−1):
-
experimental values measured
- E a (kJ mol−1):
-
apparent activation energy
- f(α) (dimensionless):
-
reaction model
- FC (wt%):
-
fixed carbon
- FR:
-
Friedman
- FWO:
-
Flynn-Wall-Ozawa
- g(α) (dimensionless):
-
integral form of the reaction model
- HHV (MJ kg−1):
-
higher heating value
- h (J s−1):
-
Plank constant
- KAS:
-
Kissinger-Akahira-Sunose
- k B (J K−1):
-
Boltzmann constant
- LHV (MJ kg−1):
-
lower heating value
- M (dimensionless):
-
total number of points used for deconvolution
- My (dimensionless):
-
mean residual
- m 0 (g):
-
initial mass
- m ∞ (g):
-
final mass
- N (dimensionless):
-
total of componets proposed for residue
- p(x) (dimensionless):
-
approximation for temperature integral equation
- PS:
-
pseudo-component
- QOF (dimensionless):
-
quality of fit
- R (kJ mol−1 K−1):
-
gas constant
- R 2 (dimensionless):
-
coefficient of determination
- RSS (dimensionless):
-
residual sum of squares
- s (dimensionless):
-
asymmetry shape parameter
- SCB:
-
smoked cigarette butts
- STK:
-
Starink
- t (min):
-
time
- T (K):
-
temperature
- TGA (wt%):
-
thermogravimetric analysis
- T m (K):
-
maximum temperature peak
- T p (K):
-
peak temperature
- VM (wt%):
-
volatile matter
- w (dimensionless):
-
half-width in the curve
- y (dimensionless):
-
residual
- α (dimensionless):
-
physical parameters of conversion
- β (K min−1):
-
heating rate
- η (dimensionless):
-
contribution of pseudo-component
- θ (dimensionless):
-
amplitude
- ΔG (kJ mol−1):
-
Gibb’s free energy change
- ΔH (kJ mol−1):
-
enthalpy change
- ΔS (J mol−1 K−1):
-
entropy change
References
Mumbach GD, Alves JLF, Da Silva JCG et al (2019) Thermal investigation of plastic solid waste pyrolysis via the deconvolution technique using the asymmetric double sigmoidal function: determination of the kinetic triplet, thermodynamic parameters, thermal lifetime and pyrolytic oil composition for clean. Energy Convers Manag 200:112031. https://doi.org/10.1016/j.enconman.2019.112031
Czajczyńska D, Anguilano L, Ghazal H, Krzyżyńska R, Reynolds AJ, Spencer N, Jouhara H (2017) Potential of pyrolysis processes in the waste management sector. Therm Sci Eng Prog 3:171–197. https://doi.org/10.1016/j.tsep.2017.06.003
Glugoski LP, de Jesus CP, Fujiwara ST (2017) Reactive Black 5 dye degradation using filters of smuggled cigarette modified with Fe3+. Environ Sci Pollut Res 24:6143–6150. https://doi.org/10.1007/s11356-016-6820-0
d’Heni Teixeira MB, Duarte MAB, Raposo Garcez L, Camargo Rubim J, Hofmann Gatti T, Suarez PAZ (2017) Process development for cigarette butts recycling into cellulose pulp. Waste Manag 60:140–150. https://doi.org/10.1016/j.wasman.2016.10.013
Sun H, La P, Yang R et al (2017) Innovative nanoporous carbons with ultrahigh uptakes for capture and reversible storage of CO2 and volatile iodine. J Hazard Mater 321:210–217. https://doi.org/10.1016/j.jhazmat.2016.09.015
Slaughter E, Gersberg RM, Watanabe K, Rudolph J, Stransky C, Novotny TE (2011) Toxicity of cigarette butts, and their chemical components, to marine and freshwater fish. Tob Control 20:i25–i29. https://doi.org/10.1136/tc.2010.040170
Soltani SM, Yazdi SK, Hosseini S, Gargari MK (2014) Effect of nitric acid modification on porous characteristics of mesoporous char synthesized from the pyrolysis of used cigarette filters. J Environ Chem Eng 2:1301–1308. https://doi.org/10.1016/j.jece.2014.04.005
Wang W, Wang M, Huang J, Li X, Cai L, Shi SQ, Cui Y, Chen L, Ni Y (2020) High efficiency pyrolysis of used cigarette filters for ester-rich bio-oil through microwave-assisted heating. J Clean Prod 257:120596. https://doi.org/10.1016/j.jclepro.2020.120596
Koochaki CB, Khajavi R, Rashidi A, Mansouri N, Yazdanshenas ME (2019) The effect of pre-swelling on the characteristics of obtained activated carbon from cigarette butts fibers. Biomass Convers Biorefinery 10:227–236. https://doi.org/10.1007/s13399-019-00429-x
Naqvi SR, Prabhakara HM, Bramer EA, Dierkes W, Akkerman R, Brem G (2018) A critical review on recycling of end-of-life carbon fibre/glass fibre reinforced composites waste using pyrolysis towards a circular economy. Resour Conserv Recycl 136:118–129. https://doi.org/10.1016/j.resconrec.2018.04.013
Qureshi MS, Oasmaa A, Pihkola H, Deviatkin I, Tenhunen A, Mannila J, Minkkinen H, Pohjakallio M, Laine-Ylijoki J (2020) Pyrolysis of plastic waste: opportunities and challenges. J Anal Appl Pyrolysis 148:104804. https://doi.org/10.1016/j.jaap.2020.104804
Naqvi SR, Uemura Y, Osman NB, Yusup S, Nuruddin MF (2014) Physiochemical properties of pyrolysis oil derived from fast pyrolysis of wet and dried rice husk in a free fall reactor. Appl Mech Mater 625:604–607. https://doi.org/10.4028/www.scientific.net/AMM.625.604
Samolada MC, Zabaniotou AA (2014) Comparative assessment of municipal sewage sludge incineration, gasification and pyrolysis for a sustainable sludge-to-energy management in Greece. Waste Manag 34:411–420. https://doi.org/10.1016/j.wasman.2013.11.003
Beneroso D, Bermúdez JM, Arenillas A, Menéndez JA (2014) Influence of the microwave absorbent and moisture content on the microwave pyrolysis of an organic municipal solid waste. J Anal Appl Pyrolysis 105:234–240. https://doi.org/10.1016/j.jaap.2013.11.009
Borges NB, Campos JR, Pablos JM (2015) Characterization of residual sand removed from the grit chambers of a wastewater treatment plant and its use as fine aggregate in the preparation of non-structural concrete. Water Pract Technol 10:164–171. https://doi.org/10.2166/wpt.2015.018
Janković B (2014) The pyrolysis of coffee paper cup waste samples using non-isothermal thermo-analytical techniques. The use of combined kinetic and statistical analysis in the interpretation of mechanistic features of the process. Energy Convers Manag 85:33–49. https://doi.org/10.1016/j.enconman.2014.05.094
Liu H, Wang C, Zhang J, Zhao W, Fan M (2020) Pyrolysis kinetics and thermodynamics of typical plastic waste. Energy Fuel 34:2385–2390. https://doi.org/10.1021/acs.energyfuels.9b04152
Aslan DI, Özoğul B, Ceylan S, Geyikçi F (2018) Thermokinetic analysis and product characterization of medium density fiberboard pyrolysis. Bioresour Technol 258:105–110. https://doi.org/10.1016/j.biortech.2018.02.126
Tahir MH, Çakman G, Goldfarb JL, Topcu Y, Naqvi SR, Ceylan S (2019) Demonstrating the suitability of canola residue biomass to biofuel conversion via pyrolysis through reaction kinetics, thermodynamics and evolved gas analyses. Bioresour Technol 279:67–73. https://doi.org/10.1016/j.biortech.2019.01.106
White JE, Catallo WJ, Legendre BL (2011) Biomass pyrolysis kinetics: a comparative critical review with relevant agricultural residue case studies. J Anal Appl Pyrolysis 91:1–33. https://doi.org/10.1016/j.jaap.2011.01.004
Yao Z, Yu S, Su W, Wu W, Tang J, Qi W (2020) Kinetic studies on the pyrolysis of plastic waste using a combination of model-fitting and model-free methods. Waste Manag Res 38:77–85. https://doi.org/10.1177/0734242X19897814
Mishra A, Kumari U, Turlapati VY, Siddiqi H, Meikap BC (2020) Extensive thermogravimetric and thermo-kinetic study of waste motor oil based on iso-conversional methods. Energy Convers Manag 221:113194. https://doi.org/10.1016/j.enconman.2020.113194
da Silva JCG, de Albuquerque JG, Galdino WV de A, et al. (2020) Single-step and multi-step thermokinetic study – deconvolution method as a simple pathway for describe properly the biomass pyrolysis for energy conversion. Energy Convers Manag 209:112653. https://doi.org/10.1016/j.enconman.2020.112653
Siddiqi H, Kumari U, Biswas S, Mishra A, Meikap BC (2020) A synergistic study of reaction kinetics and heat transfer with multi-component modelling approach for the pyrolysis of biomass waste. Energy 204:117933. https://doi.org/10.1016/j.energy.2020.117933
Hameed Z, Naqvi SR, Naqvi M, Ali I, Taqvi SAA, Gao N, Hussain SA, Hussain S (2020) A comprehensive review on thermal coconversion of biomass, sludge, coal, and their blends using thermogravimetric analysis. J Chemother 2020:1–23. https://doi.org/10.1155/2020/5024369
Naqvi SR, Ali I, Nasir S, Ali Ammar Taqvi S, Atabani AE, Chen WH (2020) Assessment of agro-industrial residues for bioenergy potential by investigating thermo-kinetic behavior in a slow pyrolysis process. Fuel 278:118259. https://doi.org/10.1016/j.fuel.2020.118259
Hameed Z, Aman Z, Naqvi SR, Tariq R, Ali I, Makki AA (2018) Kinetic and thermodynamic analyses of sugar cane bagasse and sewage sludge co-pyrolysis process. Energy Fuel 32:9551–9558. https://doi.org/10.1021/acs.energyfuels.8b01972
Fernandez A, Ortiz LR, Asensio D, Rodriguez R, Mazza G (2020) Kinetic analysis and thermodynamics properties of air/steam gasification of agricultural waste. J Environ Chem Eng 8:103829. https://doi.org/10.1016/j.jece.2020.103829
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N (2011) ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta 520:1–19. https://doi.org/10.1016/j.tca.2011.03.034
Manić N, Janković B, Pijović M, Waisi H, Dodevski V, Stojiljković D, Jovanović V (2020) Apricot kernel shells pyrolysis controlled by non-isothermal simultaneous thermal analysis (STA). J Therm Anal Calorim. https://doi.org/10.1007/s10973-020-09307-5
ASTM (2014) E1131-08: Standard test method for compositional analysis by thermogravimetry. In: Annual Book of ASTM Standards. ASTM International, West Conshohocken, pp 1– 6. https://doi.org/10.1520/E1131-08R14
ASTM (2008) D5373-08: Standard test methods for instrumental determination of carbon, hydrogen, and nitrogen in laboratory samples of coal. In: Annual Book of ASTM Standards. ASTM International, West Conshohocken, pp 1–9. https://doi.org/10.1520/D5373-08
Channiwala SA, Parikh PP (2002) A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 81:1051–1063. https://doi.org/10.1016/S0016-2361(01)00131-4
Alves JLF, Da Silva JCG, da Silva Filho VF et al (2019) Bioenergy potential of red macroalgae Gelidium floridanum by pyrolysis: evaluation of kinetic triplet and thermodynamics parameters. Bioresour Technol 291:121892. https://doi.org/10.1016/j.biortech.2019.121892
Van de Velden M, Baeyens J, Boukis I (2008) Modeling CFB biomass pyrolysis reactors. Biomass Bioenergy 32:128–139. https://doi.org/10.1016/j.biombioe.2007.08.001
Vyazovkin S, Chrissafis K, Di Lorenzo ML et al (2014) ICTAC Kinetics Committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim Acta 590:1–23. https://doi.org/10.1016/j.tca.2014.05.036
Alves JLF, Da Silva JCG, da Silva Filho VF et al (2019) Determination of the bioenergy potential of Brazilian pine-fruit shell via pyrolysis kinetics, thermodynamic study, and evolved gas analysis. Bioenergy Res 12:168–183. https://doi.org/10.1007/s12155-019-9964-1
Perejón A, Sánchez-Jiménez PE, Criado JM, Pérez-Maqueda LA (2011) Kinetic analysis of complex solid-state reactions. A new deconvolution procedure. J Phys Chem B 115:1780–1791. https://doi.org/10.1021/jp110895z
Vyazovkin S (2015) Isoconversional kinetics of thermally stimulated processes, 1st edn. Springer International Publishing, Cham
Konwar K, Nath HP, Bhuyan N, Saikia BK, Borah RC, Kalita AC, Saikia N (2019) Effect of biomass addition on the devolatilization kinetics, mechanisms and thermodynamics of a northeast Indian low rank sub-bituminous coal. Fuel 256:115926. https://doi.org/10.1016/j.fuel.2019.115926
Boonchom B (2008) Kinetics and thermodynamic properties of the thermal decomposition of manganese dihydrogenphosphate dihydrate. J Chem Eng Data 53:1533–1538. https://doi.org/10.1021/je800103w
Danvirutai C, Noisong P, Youngme S (2010) Some thermodynamic functions and kinetics of thermal decomposition of NH4MnPO4 • H2O in nitrogen atmosphere. J Therm Anal Calorim 100:117–124. https://doi.org/10.1007/s10973-009-0017-4
Boonchom B, Puttawong S (2010) Thermodynamics and kinetics of the dehydration reaction of FePO4·2H2O. Phys B Condens Matter 405:2350–2355. https://doi.org/10.1016/j.physb.2010.02.046
Mythili R, Venkatachalam P, Subramanian P, Uma D (2013) Characterization of bioresidues for biooil production through pyrolysis. Bioresour Technol 138:71–78. https://doi.org/10.1016/j.biortech.2013.03.161
Kalkreuth W, Holz M, Kern M, Machado G, Mexias A, Silva MB, Willett J, Finkelman R, Burger H (2006) Petrology and chemistry of Permian coals from the Paraná Basin: 1. Santa Terezinha, Leão-Butiá and Candiota Coalfields, Rio Grande do Sul, Brazil. Int J Coal Geol 68:79–116. https://doi.org/10.1016/j.coal.2005.10.006
Alves JLF, da Silva JCG, Mumbach GD, Domenico MD, da Silva Filho VF, de Sena RF, Machado RAF, Marangoni C (2020) Insights into the bioenergy potential of jackfruit wastes considering their physicochemical properties, bioenergy indicators, combustion behaviors, and emission characteristics. Renew Energy 155:1328–1338. https://doi.org/10.1016/j.renene.2020.04.025
Maderuelo-Sanz R, Gómez Escobar V, Meneses-Rodríguez JM (2018) Potential use of cigarette filters as sound porous absorber. Appl Acoust 129:86–91. https://doi.org/10.1016/j.apacoust.2017.07.011
Olugbade TO, Ojo OT (2020) Biomass Torrefaction for the production of high-grade solid biofuels: a review. BioEnergy Res. https://doi.org/10.1007/s12155-020-10138-3
Olugbade T, Ojo O, Mohammed T (2019) Influence of binders on combustion properties of biomass briquettes: a recent review. BioEnergy Res 12:241–259. https://doi.org/10.1007/s12155-019-09973-w
Olugbade TO, Ojo OT (2020) Binderless briquetting technology for lignite briquettes: a review. Energy, Ecol Environ. https://doi.org/10.1007/s40974-020-00165-3
Fan G, Liao C, Fang T, Luo S, Song G (2014) Amberlyst 15 as a new and reusable catalyst for the conversion of cellulose into cellulose acetate. Carbohydr Polym 112:203–209. https://doi.org/10.1016/j.carbpol.2014.05.082
Calabuig E, Juárez-Serrano N, Marcilla A (2019) TG-FTIR study of evolved gas in the decomposition of different types of tobacco. Effect of the addition of SBA-15. Thermochim Acta 671:209–219. https://doi.org/10.1016/j.tca.2018.12.006
Idris SS, Rahman NA, Ismail K (2012) Combustion characteristics of Malaysian oil palm biomass, sub-bituminous coal and their respective blends via thermogravimetric analysis (TGA). Bioresour Technol 123:581–591. https://doi.org/10.1016/j.biortech.2012.07.065
Kibet J, Kurgat C, Limo S, Rono N, Bosire J (2016) Kinetic modeling of nicotine in mainstream cigarette smoking. Chem Cent J 10:60. https://doi.org/10.1186/s13065-016-0206-8
Wang X, Wang Z, Dai Y, Ma K, Zhu L, Tan H (2017) Thermogravimetric study on the flue-cured tobacco leaf pyrolysis and combustion using a distributed activation energy model. Asia-Pacific J Chem Eng 12:75–84. https://doi.org/10.1002/apj.2055
Zhao D, Dai Y, Chen K, Sun Y, Yang F, Chen K (2013) Effect of potassium inorganic and organic salts on the pyrolysis kinetics of cigarette paper. J Anal Appl Pyrolysis 102:114–123. https://doi.org/10.1016/j.jaap.2013.03.007
Henrique MA, Flauzino Neto WP, Silvério HA, Martins DF, Gurgel LVA, Barud HS, Morais LC, Pasquini D (2015) Kinetic study of the thermal decomposition of cellulose nanocrystals with different polymorphs, cellulose I and II, extracted from different sources and using different types of acids. Ind Crop Prod 76:128–140. https://doi.org/10.1016/j.indcrop.2015.06.048
Starink MJ (2003) The determination of activation energy from linear heating rate experiments: A comparison of the accuracy of isoconversion methods. Thermochim Acta 404:163–176. https://doi.org/10.1016/S0040-6031(03)00144-8
Funding
This work was supported by Brazil’s National Council for Scientific and Technological Development (CNPq/Brazil Process 423869/2016-7) and Brazil’s Coordination for the Improvement of Higher Education Personnel (CAPES/Brazil Finance Code 001). This work was developed in the Laboratory of Activated Carbon (LCA/UFPB) and Laboratory of Control and Polymerization Processes (LCP/UFSC).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alves, J.L.F., da Silva, J.C.G., Mumbach, G.D. et al. Thermo-kinetic investigation of the multi-step pyrolysis of smoked cigarette butts towards its energy recovery potential. Biomass Conv. Bioref. 12, 741–755 (2022). https://doi.org/10.1007/s13399-020-01077-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-01077-2