Abstract
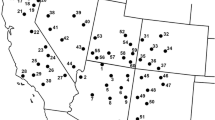
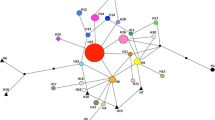
Molecular markers prove to be an invaluable tool in assessing the introduction dynamics, pattern of range expansion, and population genetics of an invasive species. Ventenata dubia (Leers) Coss. (Aveneae; ventenata) is a diploid, primarily self-pollinating, annual grass native to Eurasia and Northern Africa. The grass has a detailed herbarium collection history in the western United States since its discovery in eastern Washington in 1952. Genetic analysis of 51 invasive populations (1636 individuals) of V. dubia, coupled with historical records, suggests moderate propagule pressure from multiple introductions, followed by local or regional range expansion. Allozyme analysis detected nine multilocus genotypes (MLGs) across eight western US states. A single MLG, referred to as the most common genotype, was detected in 37 of 51 (72.5%) invasive populations across all states. The other eight MLGs were generally found in fewer populations, with limited geographic distributions. Despite multiple introductions, invasive populations exhibit low levels of genetic admixture, low levels of genetic diversity within populations (A = 1.03, %P = 2.94, Hexp = 0.007) and high genetic differentiation among populations (GST= 0.864). The apparent reduced evolutionary potential of most V. dubia populations did not preclude the initial establishment and rapid spread of this species across its new range in the western US.




Similar content being viewed by others
References
Agapow PM, Burt A (2001) Indices of multilocus linkage disequilibrium. Mol Ecol Notes 1:101–102
Alatalo RV (1981) Problems in the measurement of evenness in ecology. Oikos 37:199–204
Barrett SCH, Colautti RI, Eckert CG (2008) Plant reproductive systems and evolution during biological invasions. Mol Ecol 17:373–383
Barrett SCH (2015) Foundations of invasion genetics: the Baker and Stebbins legacy. Mol Ecol 24:1927–1941
Bellard C, Cassey P, Blackburn TM (2016) Alien species as a driver of recent extinctions. Biol Lett 12:20150623. https://doi.org/10.1098/rsb.2015.0623
Blackburn TM, Lockwood JL, Cassey P (2015) The influence of numbers on invasion success. Mol Ecol 24:1942–1953
Bossdorf O, Auge H, Lafuma L, Rogers WE, Siemann E, Prati D (2005) Phenotypic and genetic differentiation between native and introduced plant populations. Oecologia 144:1–11
Brown AHD, Burdon JJ (1987) Mating systems and colonizing success in plants. Brit Ecol Soc Symp 26:115–131
Brown AHD, Marshall DR (1981) Evolutionary changes accompanying colonization in plants. In: Scudder GGE, Reveal JL (eds) Evolution today. Carnegie-Mellon University, Pittsburg, pp 351–363
Brown ADH, Feldman MW, Nevo E (1980) Multilocus structure of natural populations of Hordeum spontaneum. Genetics 96:523–536
Butler MD (2011) Rehabilitating Ventenata infested rangelands using herbicides in conjunction with bunchgrass seedings. In: Proceedings of Western Society of weed science, vol 64, p 108
Chauvel B, Dessaint F, Cardinal-Legrand C, Bretagnolle F (2006) The historical spread of Ambrosia artemiisiifolia in France from herbarium records. J Biogeogr 33:665–673
Contu S (2013) Ventenata dubia. The IUCN Red List of Threatened Species. https://www.iucnredlist.org/species/44392189/44414263. Accessed 12 Oct 2019
D’Antonio CM, Vitousek PM (1992) Biological invasions by exotic grasses, the grass/fire cycle, and global change. Annu Rev Ecol Syst 23:63–87
Daru BH, Park DS, Primack RB et al (2018) Widespread sampling biases in herbarium reveled from large-scale digitizing. New Phytol 17:939–955
Delisle F, Lavoie C, Jean M, Lachance D (2003) Reconstructing the spread of invasive plants: taking into account biases associated with herbarium specimens. J Biogeogr 30:1033–1042
Dlugosch KM, Parker IM (2008) Founding events in species invasions: genetic variation, adaptive evolution, and the role of multiple introductions. Mol Ecol 17:431–449
Earl DA (2012) STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Resour 4:359–361
Estoup A, Guillemaud T (2010) Reconstructing routes of invasion using genetic data: why, how and so what. Mol Ecol 19:4113–4130
Estoup A, Baird SJ, Ray N, Currat M, Cornuet JM, Santos F, Beaumont MA, Excoffier L (2010) Combining genetic, historical and geographical data to reconstruct the dynamics of bioinvasions: application to the cane toad Bufo marinus. Mol Ecol Resour 10:886–901
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Flint-Garcia SA, Thornsberry JM, Buckler ES (2003) Structure of linkage disequilibrium in plants. Annu Rev Plant Biol 54:357–374
Flora of North America Editorial Committee (1993) Flora of North America: Magnoliophyta: Commelinidae (in part): Cyperaceae, vol 23. Oxford University Press, New York
Fountain B (2011) Producing timothy hay and managing for the impacts for Ventana. In: Proceedings of the Western Society of weed science, vol 64, pp 107–108
Francis RM (2017) pophelper: an R package and web app to analyse and visualize population structure. Mol Ecol Resour 17:27–32
Fryer JL (2017) Ventenata dubia. Fire Effects Information System. US Department of Agriculture, Forest Service, Rocky Mountain Research Station, Missoula Fire Sciences Laboratory (Producer). https://www.fs.fed.us/database/feis/plants/graminoid/vendub/all.pdf. Accessed 12 Oct 2019
Gaertner M, Biggs R, Beest MT, Hui C, Molofsky J, Richardson DM (2014) Invasive plants as drivers of regime shifts: identifying high-priority invaders that alter feedback relationships. Divers Distrib 20:733–744
Gaskin JF, Bon MC, Cock MJ, Cristofaro M, De Biase A, De Clerck-Floate R, Ellison CA, Hinz HL, Hufbauer RA, Julien MH, Sforza R (2011) Applying molecular based approaches to classical biological control of weeds. Biol Control 58:1–21
Gaskin JF, Schwarzlander M, Kinter CL, Smith JF, Novak SJ (2013) Propagule pressure, genetic structure, and geographic origins of Chondrilla juncea (Asteraceae): an apomictic invader on three continents. Am J Bot 100:1871–1882
Gruber B, Adamack AT (2015) Landgenreport: a new R function to simplify landscape genetic analysis using resistance surface layers. Mol Ecol Resour 15:1172–1178
Grünwald NJ, Goodwin SB, Milgroom MG, Fry WE (2003) Analysis of genotypic diversity data for populations of microorganisms. Phytopathology 93:738–746
Hamrick JL, Godt MW (1996) Effects of life history traits on genetic diversity in plant species. Philos Trans R Soc Lond B Biol Sci 351:1291–1298
Heger T, Trepl L (2003) Predicting biological invasions. Biol Invasions 5:313–321
Hufbauer RA, Facon B, Ravigné V, Turgeon J, Foucaud J, Lee CE, Rey O, Estoup A (2011) Anthropogenically induced adaptation to invade (AIAI): contemporary adaptation to human-altered habitats within the native range can promote invasions. Evol Appl 5:89–101
Huttanus TD, Mack RN, Novak SJ (2011) Propagule pressure and introduction pathways of Bromus tectorum (Cheatgrass; Poaceae) in the central United States. Int J Plant Sci 172:783–794
Kamvar ZN, Brooks JC, Grünwald NJ (2015) Novel R tools for analysis of genome-wide population genetic data with emphasis on clonality. Front Genet 6:208
Keller SR, Taylor DR (2008) History, chance and adaptation during biological invasion: separating stochastic phenotypic evolution from response to selection. Ecol Lett 11:852–866
Kolar CS, Lodge DM (2001) Progress in invasion biology: predicting invaders. Trends Ecol Evol 16:199–204
Kolbe JJ, Glor RE, Schettino LR, Lara AC, Larson A, Losos JB (2004) Genetic variation increases during biological invasion by a Cuban lizard. Nature 431:177–181
Lockwood JL, Cassey P, Blackburn T (2005) The role of propagule pressure in explaining species invasions. Trends Ecol Evol 20:223–228
Ludwig JA, Reynolds JF (1988) Statistical ecology: a primer in methods and computing, vol 1. Wiley, New York, pp 94–95
Nei M (1978) Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89:583–590
Nei M (1987) Molecular evolutionary genetics. Columbia University Press, New York
Nei M, Chesser RK (1983) Estimation of fixation indices and gene diversities. Ann Hum Genet 47:253–259
Novak SJ (2007) The role of evolution in the invasion process. Proc Natl Acad Sci 104:3671–3672
Novak S (2011) Geographic origins and introduction dynamics. In: Simberloff D, Rejmanek M (eds) Encyclopedia of biological invasions. University of California Press, Berkeley, pp 272–280
Novak SJ, Mack RN (2001) Tracing plant introduction and spread: genetic evidence from Bromus tectorum (Cheatgrass). Bioscience 51:114–122
Novak S, Mack R (2005) Genetic bottlenecks in alien plant species: influence of mating systems and introduction dynamics. In: Sax DF, Stachowicz JJ, Gaines SD (eds) Species invasions: insights into ecology, evolution, and biogeography. Sinauer Associates Inc, Sunderland, pp 201–228
Novak SJ, Mack RN (2016) Mating system, introduction and genetic diversity of Bromus tectorum in North America, the most notorious product of evolution within Bromus section Genea. In: Germino MJ, Chambers JC, Brown CS (eds) Exotic brome-grasses in arid and semiarid ecosystems of the Western US. Springer, Cham, pp 99–132
Novak SJ, Mack RN, Soltis DE (1991) Genetic variation in Bromus tectorum (Poaceae): population differentiation in its North American range. Am J Bot 78:1150–1161
Olafsson K, Pampoulie C, Hjorleifsdottir S, Gudjonsson S, Hreggvidsson GO (2014) Present-day genetic structure of Atlantic salmon (Salmo salar) in Icelandic rivers and ice-cap retreat models. PLoS ONE 9:86809
Pannell JR (2015) Evolution of the mating system in colonizing plants. Mol Ecol 24:2018–2037
Pavek P, Wallace J, Prather T (2011) Ventenata biology and distribution in the Pacific Northwest. In: Proceedings of Western Society of weed science, vol 64, pp 07–107
Pawlak AR, Mack RN, Busch JW, Novak SJ (2015) Invasion of Bromus tectorum (L.) into California and the American Southwest: rapid, multi-directional and genetically diverse. Biol Invasions 17:287–306
Pielou EC (1966) Shannon’s formula as a measure of specific diversity: its use and misuse. Am Nat 100:463–465
Pimentel D, Zuniga R, Morrison D (2005) Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol Econ 52:273–288
Prather T (2009) Ventenata dubia: Increasing concern to the Inland Northwest Region. Center for Invasive Plant Management, June, 1–2
Prather T (2018) Ventenata dubia (North Africa grass). CABI. Invasive Species Compendium https://www.cabi.org/isc/datasheet/117772. Accessed 12 Oct 2019
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Rey O, Estoup A, Vonshak M, Loiseau A, Blanchet S, Calcaterra L, Chifflet L, Rossi JP, Kergoat GJ, Foucaud J, Orivel J (2012) Where do adaptive shifts occur during invasion? A multidisciplinary approach to unravelling cold adaptation in a tropical ant species invading the Mediterranean area. Ecol Lett 15:1266–1275
Ricciardi A, Jones LA, Kestrup AM, Ward JM (2011) Expanding the propagule pressure concept to understand the impact of biological invasions. In: Richardson DM (ed) Fifty years of invasion ecology: the legacy of Charles Elton. Wiley-Blackwell, Hoboken, pp 225–235
Rowe G, Sweet M, Beebee T (2017) An introduction to molecular ecology. Oxford University Press, Oxford
Salo LF (2005) Red brome (Bromus rubens subsp. madritensis) in North America: possible modes for early introductions, subsequent spread. Biol Invasions 7:165–180
Simberloff D (2009) The role of propagule pressure in biological invasions. Annu Rev Ecol Evol Syst 40:81–102
Simberloff D, Martin JL, Genovesi P, Maris V, Wardle DA, Aronson J, Courchamp F, Galil B, García-Berthou E, Pascal M, Pyšek P (2013) Impacts of biological invasions: what’s what and the way forward. Trends Ecol Evol 28:58–66
Simpson E (1949) Measurement of diversity. Nature 163:688
Soltis DE, Haufler CH, Darrow DC, Gastony GL (1983) Starch gel electrophoresis of ferns: a compilation of grinding buffers, gel and electrode buffers, and staining schedules. Am Fern J 73:9–27
Taylor DR, Keller SR (2007) Historical range expansion determines the phylogenetic diversity introduced during contemporary species invasion. Evolution 61:334–345
The Plant List (2013) Ventenata dubia. http://www.theplantlist.org/. Accessed 12 Oct 2019
Vähä JP, Erkinaro J, Niemelä E, Primmer CR (2007) Life-history and habitat features influence the within-river genetic structure of Atlantic salmon. Mol Ecol 16:2638–2654
Wallace JM, Pavek PL, Prather TS (2015) Ecological characteristics of Ventenata dubia in the Intermountain Pacific Northwest. Invas Plant Sci Mana 8:57–71
Wares JP, Hughes AR, Grosberg RK (2005) Mechanisms that drive evolutionary change. Insights from species introduction and invasions. In: Sax DF, Stachowicz JJ, Gaines SD (eds) Species invasions: insights into ecology, evolution, and biogeography. Sinauer Associates, Sunderland, pp 229–257
Wilson JR, Dormontt EE, Prentis PJ, Lowe AJ, Richardson DM (2009) Something in the way you move: dispersal pathways affect invasion success. Trends Ecol Evol 24:136–144
Winter DJ (2012) MMOD: an R library for the calculation of population differentiation statistics. Mol Ecol Resour 12:1158–1160
Wright S (1965) The interpretation of population structure by F-statistics with special regard to systems of mating. Evolution 19:395–420
Yeh FC, Boyle TJB (1997) Population genetic analysis of co-dominant and dominant marker and quantitative traits. Belg J Bot 130:129–157
Acknowledgements
We thank Joe Rausch, Jerry Galland, Elizabeth Kent, Pete Guerdan, Jane Mangold, Brian Mealor, and Steve Ripple for invaluable assistance and efforts in obtaining seed collections of invasive populations of ventenata from across the western US; Ester Hotova, Theodore “TJ” Halone, and Angela Fairbanks for their unwavering laboratory assistance; Nina Gillard, Gordon, Gordie and Teddy Smith, for their patience and support. We also thank Drs. Dorothy Maguire and Tim Prather for their advice and assistance, especially during the early stages of this project. Funding for this research was provided by funding through the USDA-ARS Area-wide Pest Management Program, and this research received additional funding support through a Specific Collaborative Agreement (SCA) with the USDA-ARS, European Biological Control Laboratory, Montferrier-sur-Lez, France (0212-22000-028-02S). We are grateful to Dr. Lincoln Smith for his support of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pervukhina-Smith, I., Sforza, R.F.H., Cristofaro, M. et al. Genetic analysis of invasive populations of Ventenata dubia (Poaceae): an assessment of propagule pressure and pattern of range expansion in the Western United States. Biol Invasions 22, 3575–3592 (2020). https://doi.org/10.1007/s10530-020-02341-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-020-02341-2