Abstract
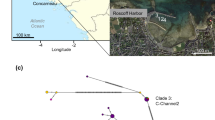
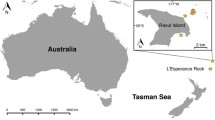
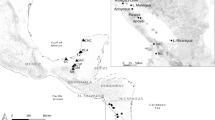
The spionid polychaete Pseudopolydora paucibranchiata (Okuda, 1937) was originally described from Japan and has since been reported as a non-indigenous species in soft bottom communities in the Northeast Pacific, the Mediterranean Sea, around Europe, Australia, Brazil, and Florida. The diagnostic features of the adults are palps with ramified yellow chromatophores, prostomium rounded anteriorly, short occipital antenna on the caruncle, and a small disc-like pygidium. We collected Pseudopolydora with these features from locations worldwide and compared them by a molecular analysis. The Bayesian analysis of the combined dataset of three genetic markers (mitochondrial 16S rDNA, nuclear 28S rDNA and Histone 3; 811 bp in total) showed that the worms form a monophyletic group comprising four genetically different clades. We name this group the P. paucibranchiata complex and consider the clades as four pseudocryptic species. The largest examined clade comprises individuals from the Pacific Canada (British Columbia), Russia (Sea of Japan), South Korea (East Sea), Italy (Tyrrhenian and Ionian Seas), Australia (Victoria), Netherlands, and Japan, which we identify as P. paucibranchiata. The morphology, reproductive biology and ecology of P. paucibranchiata are briefly reviewed. The other three clades are referred to as Pseudopolydora vexillosa Radashevsky and Hsieh, 2000 (Vietnam, Nha Trang Bay), Pseudopolydora sp. A (Australia, Northern Territory), and Pseudopolydora sp. B (Kuwait, Arabian Gulf). We explain the occurrence of P. paucibranchiata outside of the Northwest Pacific by unintentional human-mediated transportation in ballast water and/or with commercial oyster movements in aquaculture operations, followed by successful invasions.





Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Abdelnaby FA (2019) New recorded alein polydorid species (Polychaeta: Spionidae) from the Egyptian waters. Egypt J Aquat Biol Fish 23:409–420
Abe H, Kondoh T, Sato-Okoshi W (2016) First report of the morphology and rDNA sequences of two Pseudopolydora species (Annelida: Spionidae) from Japan. Zool Sci (Tokyo) 33:650–658. https://doi.org/10.2108/zs160082
Abe H, Takeuchi T, Taru M, Sato-Okoshi W, Okoshi K (2019a) Habitat availability determines distribution patterns of spionid polychaetes (Annelida: Spionidae) around Tokyo Bay. Mar Biodivers Rec 12:1–12. https://doi.org/10.1186/s41200-019-0167-4
Abe H, Tomioka S, Kobayashi G, Itoh H (2019b) Spionidae (Annelida) from Rishiri Island, northern Japan. Rishiri Stud 38:15–27
Annenkova NP (1937) The Polychaeta fauna of the northern part of the Japan Sea. Explor Mers d'USSR 23:139–216
Barnard JL, Reish DJ (1959) Ecology of Amphipoda and Polychaeta of Newport Bay, California. Allan Hancock Found Publ Occ Pap 21:1–106
Bastrop R, Jürss K, Sturmbauer C (1998) Cryptic species in a marine polychaete and their independent introduction from North America to Europe. Mol Biol Evol 15:97–103
Berge JA, Valderhaug VA (1983) Effect of epibenthic macropredators on community structure in subtidal organically enriched sediment in the inner Oslofjord. Mar Ecol Prog Ser 11:15–22
Berkeley C (1967) A checklist of Polychaeta recorded from British Columbia since 1923, with references to name changes, descriptions, and synonymies. I. Errantia. Can J Zool 45:1049–1059
Berkeley C (1968) A checklist of Polychaeta recorded from British Columbia since 1923, with references to name changes, descriptions, and synonymies. II. Sedentaria. Can J Zool 46:557–567
Berkeley E, Berkeley C (1935) Some notes on the polychaetous annelids at Elkhorn Slough, Monterey Bay, California. Am Midl Nat 16:766–775
Berkeley E, Berkeley C (1936) Notes on Polychaeta from the coast of western Canada. I. Spionidae. Ann Mag Nat Hist Lond Ser 10(18):468–477
Berkeley E, Berkeley C (1942) North Pacific Polychaeta, chiefly from the west coast of Vancouver Island, Alaska, and Bering Sea. Can J Res 20(Sec. D):183–208. https://doi.org/10.1139/cjr42d-016
Berkeley E, Berkeley C (1948) Canadian Pacific Fauna. Annelida. Polychaeta Errantia. J Fish Res Board Can 9b:1–100
Berkeley E, Berkeley C (1952) Canadian Pacific Fauna. Annelida. Polychaeta Sedentaria. J Fish Res Board Can 9:1–139
Blake JA (1975) Phylum Annelida: Class Polychaeta. In: Smith RI, Carlton JT (eds) Light’s Manual: Intertidal Invertebrates of the Central California Coast. University of California Press, Berkeley, pp 151–243
Blake JA (2006) Spionida. In: Rouse G, Pleijel F (eds) Reproductive biology and phylogeny of Annelida, vol 4. Series: reproductive biology and phylogeny. Science Publisher, Enfield, pp 565–638
Blake JA, Kudenov JD (1978) The Spionidae (Polychaeta) from southeastern Australia and adjacent areas with a revision of the genera. Mem Natl Mus Vic 39:171–280
Blake JA, Ruff RE (2007) Annelida: Polychaeta. In: Carlton JT (ed) The Light & Smith manual: intertidal invertebrates from central California to Oregon. University of California Press, Berkeley, pp 309–410
Blake JA, Woodwick KH (1975) Reproduction and larval development of Pseudopolydora paucibranchiata (Okuda) and Pseudopolydora kempi (Southern) (Polychaeta: Spionidae). Biol Bull 149:109–127. https://doi.org/10.2307/1540483
Blake JA, Maciolek NJ, Meißner K (2020) Spionidae Grube, 1850. In: Purschke G, Böggemann M, Westheide W (eds) Handbook of Zoology: Annelida Volume 2: Pleistoannelida, Sedentaria II. De Gruyter, Berlin, pp 1–103
Blank M, Bastrop R (2009) Phylogeny of the mud worm genus Marenzelleria (Polychaeta, Spionidae) inferred from mitochondrial DNA sequences. Zool Scr 38:313–321. https://doi.org/10.1111/j.1463-6409.2008.00370.x
Bogantes VE, Halanych KM, Meißner K (2018) Diversity and phylogenetic relationships of North Atlantic Laonice Malmgren, 1867 (Spionidae, Annelida) including the description of a novel species. Mar Biodivers 48:737–749. https://doi.org/10.1007/s12526-018-0859-8
Bogantes VE, Halanych KM, Boyle MJ (2019) Larval development of Pseudopolydora paucibranchiata (Spionidae, Annelida) from Florida. In: Pernet B, Fitzhugh K, Harris L, Lovell L, Whitcraft C (eds) 13th international polychaete conference, Los Angeles, California, 4–9 August 2019, Program & Abstracts. https://nhm.org/sites/default/files/2019-07/ipc13_program_final_26jul19.pdf, p 24
Boudouresque CF (2005) Les espèces introduites et invasives en milieu marin, Deuxième édn. GIS Posidonie publ, Marseilles
Britayev TA, Rzhavsky AV (1985) Contribution to the fauna of polydorids (Polychaeta, Spionidae) from the Sea of Japan. Bull Moscow Soc Nat 90:45–50
Buzhinskaja GN (1985) Polychaeta of the shelf off south Sakhalin and their ecology. Explor Fauna Seas 30:72–224
Carlton JT (1975) Introduced intertidal invertebrates. In: Smith RI, Carlton JT (eds) University of California Press, Berkeley, pp 17–25
Carlton JT (1979) History, biogeography, and ecology of the introduced marine and estuarine invertebrates of the Pacific coast of North America. Ph.D. Dissertation, Ecology. Davis, University of California
Carlton JT (2001) Introduced Species in U.S. Coastal Waters: Environmental Impacts and Management Priorities. Pew Oceans Commission, Arlington, TX
Carlton JT (2009) Deep invasion ecology and the assembly of communities in historical time. In: Rilov G, Crooks JA (eds) Biological invasions in marine ecosystems ecological studies 204. Springer, Berlin, pp 13–56
Carlton JT, Ruiz GM (2005) The magnitude and consequences of bioinvasions in marine ecosystems: implications for conservation biology. In: Norse EA, Crowder LB (eds) Marine Conservation Biology: The Science of Maintaining the Sea’s Biodiversity. Island Press, Washington, pp 123–148
Carlton JT, Reid DM, van Leeuwen H (1995) Shipping Study. The Role of Shipping in the Introduction of Non-indigenous Aquatic Organisms to the Coastal Waters of the United States (Other Than the Great Lakes) and an Analysis of Control Options. Report Number CG- D-11-95. Government Accession Number AD-A294809, The National Sea Grant College Program/Connecticut Sea Grant Project R/ES-6. Department of Transportation, United States Coast Guard, Washington, DC, Groton, CT
Castresana J (2000) Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol 17:540–552
Chandramouli KH, Mok FSY, Wang H, Qian P-Y (2011) Phosphoproteome analysis during larval development and metamorphosis in the spionid polychaete Pseudopolydora vexillosa. BMC Dev Biol 11:1–15
Çinar ME, Dagli E (2013) Polychaetes (Annelida: Polychaeta) from the Aegean and Levantine coasts of Turkey, with descriptions of two new species. J Nat Hist Lond 47:911–947. https://doi.org/10.1080/00222933.2012.752543
Çinar ME, Dagli E, Açik S (2011a) Annelids (Polychaeta and Oligochaeta) from the Sea of Marmara, with descriptions of five new species. J Nat Hist, London 45:2105–2143
Çinar ME, Bilecenoglu M, Ozturk B, Katagan T, Yokes MB, Aysel V, Dagli E, Açik S, Ozcan T, Erdogan H (2011b) An updated review of alien species on the coasts of Turkey. Mediterr Mar Sci 12:257–315. https://doi.org/10.12681/mms.34
Çinar ME, Katagan T, Öztürk B, Bakir K, Dagli E, Açik S, Dogan A, Bitlis B (2012a) Spatio-temporal distributions of zoobenthos in soft substratum of Izmir Bay (Aegean Sea, eastern Mediterranean), with special emphasis on alien species and ecological quality status. J Mar Biol Assoc UK 92:1457–1477. https://doi.org/10.1017/S0025315412000264
Çinar ME, Katagan T, Öztürk B, Dagli E, Açik S, Bitlis B, Bakir K, Dogan A (2012b) Spatio-temporal distributions of zoobenthos in Mersin Bay (Levantine Sea, eastern Mediterranean) and the importance of alien species in benthic communities. Mar Biol Res 8:954–968. https://doi.org/10.1080/17451000.2012.706305
Claparède E (1868) Les Annélides Chétopodes du Golfe de Naples. Ramboz et Schuchardt, Genève. https://doi.org/10.5962/bhl.title.105355
Clark RB, Jones ML (1955) Two new Nephtys (Annelida, Polychaeta) from San Francisco Bay. J Wash Acad Sci 45:143–146
Dagli E, Çinar ME (2008) Invasion of polluted soft substratum of Izmir Bay (Aegean Sea, eastern Mediterranean) by the spionid polychaete worm, Pseudopolydora paucibranchiata (Polychaeta: Spionidae). Cah Biol Mar 49:87–96
Dagli E, Çinar ME, Ergen Z (2011) Spionidae (Annelida: Polychaeta) from the Aegean Sea (eastern Mediterranean). Ital J Zool 78:49–64
D’Alessandro M, Castriota L, Maggio T, Nasi F, Carletti M, Auriemma R, Romeo T, Del Negro P (2019) Spiophanes adriaticus, a new species from the Mediterranean Sea. J Mar Biol Assoc. https://doi.org/10.1017/S0025315419001061
Darling JA, Carlton JT (2018) A framework for understanding marine cosmopolitanism in the anthropocene. Front Mar Sci 5:1–25. https://doi.org/10.3389/fmars.2018.00293
Dyakov BS (2006) On water circulation in the Tatar Strait in spring. Izv (Bull) TINRO 146:205–212
Eleftheriou A (1970) Notes on the polychaete Pseudopolydora pulchra (Carazzi) from British waters. Cah Biol Mar 11:459–474
Faasse M (2016) Dispersal of the invasive tubeworms Desdemona ornata and Pseudopolydora paucibranchiata to the Netherlands (Polychaeta: Sedentaria). Ned Faun Meded 46:49–56
Guggolz T, Meißner K, Schwentner M, Brandt A (2019) Diversity and distribution of Laonice species (Annelida: Spionidae) in the tropical North Atlantic and Puerto Rico Trench. Sci Rep 9:1–12. https://doi.org/10.1038/s41598-019-45807-7
Hartman O (1936a) Polychaetous annelids of the littoral zone of California. Ph.D. dissertation, Zoology. University of California, Berkeley
Hartman O (1936b) New species of Spionidae (Annelida Polychaeta) from the coast of California. Univ Calif Publ Zool 41:45–52
Hartman O (1940) Boccardia proboscidea, a new species of spionid worm from California. J Wash Acad Sci 30:382–387
Hartman O (1941) Some contributions to the biology and life history of Spionidae from California. With keys to species and genera and descriptions of two new forms. Allan Hancock Pac Exped 7:289–323
Hartman O (1954) The marine annelids of San Francisco Bay and its environs, California. Allan Hancock Found Publ Occ Pap 15:1–20
Hartman O (1959) Catalogue of the Polychaetous Annelids of the World. Allan Hancock Found Publ Occ Pap 23:1–628
Hewitt CL, Campbell ML, Thresher RE, Martin RB, Boyd S, Cohen BF, Currie DR, Gomon MF, Keough MJ, Lewis JA, Lockett MM, Mays N, McArthur MA, O’Hara TD, Poore GCB, Ross DJ, Storey MJ, Watson JE, Wilson RS (2004) Introduced and cryptogenic species in Port Phillip Bay, Victoria, Australia. Mar Biol (Berlin) 144:183–202. https://doi.org/10.1007/s00227-003-1173-x
Hewitt CL, Campbell ML, Schaffelke B (2007) Introductions of seaweeds: accidental transfer pathways and mechanism. Bot Mar 50:326–337
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogeny. Bioinformatics 17:754–755. https://doi.org/10.1093/bioinformatics/17.8.754
Hutchings P, Kupriyanova E (2018) Cosmopolitan polychaetes—fact or fiction? Personal and historical perspectives. Invertebr Syst 32:1–9. https://doi.org/10.1071/IS17035
Imajima M, Hartman O (1964) The polychaetous annelids of Japan. Part II. Allan Hancock Found Publ Occ Pap 26:236–452
Jones ML (1954) The Richmond shoreline survey. State of California, Department of Public Health, Report of the Department of Fish and Game Project #54-2-3
Jones ML (1961) A quantitative evaluation of the benthic fauna off Point Richmond, California. Univ Calif Publ Zool 67:219–320
Junqueira AOR, Tavares MDS, Schaeffer-Novelli Y, Radashevsky VI, Cirelli JO, Julio LM, Romagnoli FC, dos Santos KC, Ferreira-Silva MAG (2009) Capítulo 6—Zoobentos. In: Lopes RM (ed) Informe sobre as espécies exóticas invasoras marinhas no Brasil. MMA/SBF, Brasília, pp 145–371
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780. https://doi.org/10.1093/molbev/mst010
Katoh K, Misawa K, Kuma K-i, Miyata T (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res 30:3059–3066. https://doi.org/10.1093/nar/gkf436
Kesäniemi JE, Rawson PD, Lindsay SM, Knott KE (2012) Phylogenetic analysis of cryptic speciation in the polychaete Pygospio elegans. Ecol Evol 2:994–1007
Kim IH, Sikorski A, O’Reilly M, Boxshall GA (2013) Copepods associated with polychaete worms in European Seas. Zootaxa 3651:1–62. https://doi.org/10.11646/zootaxa.3651.1.1
Kurt G, Kuş S (2019) Polychaete (Annelida) species of Golden Horn estuary (Sea of Marmara). In: Özcan T (ed) International Biodiverstiy & Ecology Sciences Symposium Proceeding (Bioeco2019), 26–28 September 2019, İstanbul/Turkey. Palas Academic Organization and Trade Corporation, İskenderun-Hatay, pp 247–251
Langeneck J, Lezzi M, Del Pasqua M, Musco L, Gambi MC, Castelli A, Giangrande A (2020) Non-indigenous polychaetes along the coasts of Italy: a critical review. Mediterr Mar Sci 21:238–275. https://doi.org/10.12681/mms.21860
Light WJ (1977) Spionidae (Annelida: Polychaeta) from San Francisco Bay, California: a revised list with nomenclatural changes, new records, and comments on related species from the northeastern Pacific Ocean. Proc Biol Soc Wash 90:66–88
Light WJ (1978) Spionidae (Polychaeta, Annelida). The Boxwood Press, Pacific Grove, California
MacGinitie GE (1935) Ecological aspects of a California marine estuary. Am Midl Nat 16:629–765
Maggiore F, Keppel E (2007) Biodiversity and distribution of polychaetes and molluscs along the Dese estuary (Lagoon of Venice, Italy). Hydrobiologia 588:189–203
Mahon AR, Mahon HK, Dauer DM, Halanych KM (2009) Discrete genetic boundaries of three Streblospio (Spionidae, Annelida) species and the status of S. shrubsolii. Mar Biol Res 5:172–178. https://doi.org/10.1080/17451000802317683
Meißner K, Blank M (2009) Spiophanes norrisi sp. nov. (Polychaeta: Spionidae)—a new species from the NE Pacific coast, separated from the Spiophanes bombyx complex based on both morphological and genetic studies. Zootaxa 2278:1–25. https://doi.org/10.5281/zenodo.191147
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the gateway computing environments workshop (GCE), 14 Nov 2010. IEEE, New Orleans, LA, pp 1–8
Minchin D, Gollasch S (2002) Vectors—How exotics get around. In: Leppäkoski E, Gollasch S, Olenin S (eds) Invasive Aquatic species of europe: distribution, impacts and management. Kluwer Academic Publishers, Dordrecht, pp 183–192
Mincks SL, Dyal PL, Paterson GLJ, Smith CR, Glover AG (2009) A new species of Aurospio (Polychaeta, Spionidae) from the Antarctic shelf, with analysis of its ecology, reproductive biology and evolutionary history. Mar Ecol 30:181–197
Mok F, Thiyagarajan V, Qian P-Y (2008) Larval development and metamorphic behaviour of the subtropical spionid polychaete Pseudopolydora vexillosa. J Exp Mar Biol Ecol 357:99–108. https://doi.org/10.1016/j.jembe.2007.12.029
Mok FSY, Thiyagarajan V, Qian P-Y (2009) Proteomic analysis during larval development and metamorphosis of the spionid polychaete Pseudopolydora vexillosa. Proteome Sci 7:1–11
Myohara M (1980) Reproduction and development of Pseudopolydora paucibranchiata (Polychaeta: Spionidae) under laboratory conditions, with special regard to the polar lobe formation. J Fac Sci Hokkaido Imp Univ Ser VI Zool 22:145–155
Nei M, Kumar S (2000) Molecular evolution and phylogenetics. Oxford University Press, Oxford. https://doi.org/10.1046/j.1365-2540.2001.0923a.x
Nikitin AA, Danchenkov MA, Lobanov VB, Yurasov GI (2009) Surface circulation and synoptic eddies in the Japan Sea. Izv (Bull) TINRO 157:158–167
Nishizawa R, Sato M, Furota T, Tosuji H (2014) Cryptic invasion of northeast Pacific estuaries by the Asian polychaete, Hediste diadroma (Nereididae). Mar Biol (Berlin) 161:187–194
Nygren A (2013) Cryptic polychaete diversity: a review. Zool Scr 43:172–183. https://doi.org/10.1111/zsc.12044
Nylander JAA (2004) MrModeltest v2. Program distributed by the author. Uppsala University, Uppsala
Okuda S (1937) Spioniform polychaetes from Japan. J Fac Sci Hokkaido Imp Univ Ser VI Zool 5:217–254
Olsgard F (1999) Effects of copper contamination on recolonization of subtidal marine soft sediments—and experimental field study. Mar Pollut Bull 38:448–462. https://doi.org/10.1016/S0025-326X(98)90202-8
Paik E-I (1989) Illustrated encyclopedia of fauna & flora of Korea, vol 31. Polychaeta, Seoul
Perdicaro R, Magliocchetti-Lombi P, Giangrande A (1980) Ricerche sul lago di Sabaudia: considerazioni a seguito della crisi distrofica verificatasi nel luglio 1979. Quad Ist Idrobiol Acquacolt ‘G Brunelli’ 1:3–23
Radashevsky VI (1983) Reproduction and larval development of the polychaete Pseudopolydora paucibranchiata (Spionidae) in Peter the Great Bay of the Sea of Japan. Biol Morya Vladivostok 38–46
Radashevsky VI (1993) Revision of the genus Polydora and related genera from the North West Pacific (Polychaeta: Spionidae). Publ Seto Mar Biol Lab 36:1–60
Radashevsky VI (2004) Polychaetes—Distribution through man’s activities. Anais do II Simpósio Brasileiro de Oceanografia, Instituto Oceanografico da Universidade de São Paulo. Universidade de São Paulo, São Paulo
Radashevsky VI (2015) Spionidae (Annelida) from Lizard Island, Great Barrier Reef, Australia: the genera Aonides, Dipolydora, Polydorella, Prionospio, Pseudopolydora, Rhynchospio, and Tripolydora. Zootaxa 4019:635–694. https://doi.org/10.11646/zootaxa.4019.1.22
Radashevsky VI, Hsieh HL (2000) Pseudopolydora (Polychaeta: Spionidae) species from Taiwan. Zool Stud 39:218–235
Radashevsky VI, Pankova VV (2013) Shell-boring versus tube-dwelling: is the mode of life fixed or flexible? Two cases in spionid polychaetes (Annelida, Spionidae). Mar Biol (Berlin) 160:1619–1624. https://doi.org/10.1007/s00227-013-2214-8
Radashevsky VI, Neretina TV, Pankova VV, Tzetlin AB, Choi J-W (2014) Molecular identity, morphology and taxonomy of the Rhynchospio glutaea complex with a key to Rhynchospio species (Annelida, Spionidae). Syst Biodivers 12:424–433. https://doi.org/10.1080/14772000.2014.941039
Radashevsky VI, Pankova VV, Neretina TV, Stupnikova AN, Tzetlin AB (2016a) Molecular analysis of the Pygospio elegans group of species (Annelida: Spionidae). Zootaxa 4083:239–250. https://doi.org/10.11646/zootaxa.4083.2.4
Radashevsky VI, Malyar VV, Pankova VV, Nuzhdin SV (2016b) Molecular analysis of six Rhynchospio Hartman, 1936 species (Annelida: Spionidae) with comments on the evolution of brooding within the group. Zootaxa 4127:579–590. https://doi.org/10.11646/zootaxa.4127.3.10
Ramberg JP, Schram TA (1983) A systematic review of the Oslofjord species of Polydora Bosc and Pseudopolydora Czerniavsky, with some new biological and ecological data (Polychaeta: Spionidae). Sarsia 68:233–247
Reish DJ (1975) Invertebrates, especially benthic annelids in outer Anaheim Bay. Fish Bull Calif Dep Fish Game 165:73–78
Rice SA (1992) Polychaeta: spermatogenesis and spermiogenesis. In: Harrison FW, Gardiner SL (eds) Microscopic Anatomy of Invertebrates, vol 7. Annelida. Wiley-Liss Inc, New York, pp 129–151
Ronquist F, Huelsenbeck JP (2003) MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574. https://doi.org/10.1093/bioinformatics/btg180
Ruiz GM, Fofonoff PW, Carlton JT, Wonham MJ, Hines AH (2000) Invasion of coastal marine communities in North America: apparent patterns, processes, and biases. Annu Rev Ecol Syst 31:481–531
Ruiz GM, Fofonoff PW, Steves B, Foss SF, Shiba SN (2011) Marine invasion history and vector analysis of California: a hotspot for western North America. Divers Distrib 17:362–373
Sato-Okoshi W (2000) Polydorid species (Polychaeta: Spionidae) in Japan, with descriptions of morphology, ecology and burrow structure. 2. Non-boring species. J Mar Biol Assoc UK 80:443–456
Sato-Okoshi W, Abe H (2012) Morphological and molecular sequence analysis of the harmful shell boring species of Polydora (Polychaeta: Spionidae) from Japan and Australia. Aquaculture 368–369:40–47. https://doi.org/10.1016/j.aquaculture.2012.08.046
Sato-Okoshi W, Abe H (2013) Morphology and molecular analysis of the 18S rRNA gene of oyster shell borers, Polydora species (Polychaeta: Spionidae), from Japan and Australia. J Mar Biol Assoc UK 93:1279–1286. https://doi.org/10.1017/s002531541200152x
Sato-Okoshi W, Abe H, Nishitani G, Simon CA (2017) And then there was one: Polydora uncinata and Polydora hoplura (Annelida: Spionidae), the problematic polydorid pest species represent a single species. J Mar Biol Assoc UK 97:1675–1684. https://doi.org/10.1017/s002531541600093x
Scholin CA, Herzog M, Sogin M, Anderson DM (1994) Identification of group- and strain-specific genetic markers for globally distributed Alexandrium (Dinophyceae). II. Sequence analysis of a fragment of the LSU rRNA gene. J Phycol 30:999–1011
Simboura N, Kurt Sahin G, Panagoulia A, Katsiaras N (2010) Four new alien species on the coasts of Greece (Eastern Mediterranean). Mediterr Mar Sci 11:341–352
Simon CA, Sato-Okoshi W, Abe H (2019a) Hidden diversity within the cosmopolitan species Pseudopolydora antennata (Claparède, 1869) (Spionidae: Annelida). Mar Biodivers 49:25–42. https://doi.org/10.1007/s12526-017-0751-y
Simon CA, Williams L-G, Henninger T (2019b) A new species of Rhynchospio (Annelida: Spionidae) in South Africa. Mar Biodivers 49:663–672. https://doi.org/10.1007/s12526-017-0842-9
Stschapova TF, Mokyevsky OB, Pasternak FA (1957) Flora and fauna of coastal zones of Putjatin Island (Sea of Japan). Part 1. Qualitative composition. Proc Inst Oceanol Acad Sci USSR 23:67–101
Sun DY (1994) Annelida: Polychaeta. In: Zong-guo H (ed) Marine species and their distributions in China’s seas. China Ocean Press, Beijing, pp 343–377
Syomin V, Sikorski A, Bastrop R, Köhler N, Stradomsky B, Fomina E, Matishov D (2017) The invasion of the genus Marenzelleria (Polychaeta: Spionidae) into the Don River mouth and the Taganrog Bay: morphological and genetic study. J Mar Biol Assoc UK 97:975–984. https://doi.org/10.1017/s0025315417001114
Takata N, Noguchi D, Tanaka M, Awakihara H (2011) Genetic characteristics of Polydora cornuta complex (Spionidae; Polychaeta) inhabiting an eutrophic port of Fukuyama. Eco-Engineering 23:101–104
Tamaki A (1984) Structural characteristics of an intertidal sand flat in Tomioka Bay, Amakusa, west Kyushu. Publ Amakusa Mar Biol Lab, Kyushu Univ 7:125–150
Tamaki A (1985) Inhibition of larval recruitment of Armandia sp. (Polychaeta: Opheliidae) by established adults of Pseudopolydora paucibranchiata (Okuda) (Polychaeta: Spionidae) on an intertidal sand flat. J Exp Mar Biol Ecol 87:67–82. https://doi.org/10.1016/0022-0981(85)90193-5
Tamaki A (1987) Comparison of resistivity to transport by wave action in several polychaete species on an intertidal sand flat. Mar Ecol Prog Ser 37:181–189
Tamaki A, Kikuchi T (1983) Spatial arrangement of macrobenthic assemblages on an intertidal sand flat, Tomioka Bay, west Kyushu. Publ Amakusa Mar Biol Lab, Kyushu Univ 7:41–60
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. https://doi.org/10.1093/molbev/msr121
Teramoto W, Sato-Okoshi W, Abe H, Nishitani G, Endo Y (2013) Morphology, 18S rRNA gene sequence and life history of a new Polydora species (Polychaeta: Spionidae) from northeastern Japan. Aquat Biol 18:31–45. https://doi.org/10.3354/ab00485
Tuck ID, Hall SJ, Robertson MR, Armstrong E, Basford DJ (1998) Effects of physical trawling disturbance in a previously unfished sheltered Scottish sea loch. Mar Ecol Prog Ser 162:227–242. https://doi.org/10.3354/meps162227
UKNBN (2020) UKNBN (United Kingdom National Biodiversity Network), https://species.nbnatlas.org/. Accessed 27 Apr 2020
Uschakov PV (1950) Polychaetes from the Sea of Okhotsk. Explor Far East Seas USSR 2:140–237
Uschakov PV (1953) The fauna of the Okhotsk Sea and conditions of their existence. USSR Academy of Sciences Press, Moscow-Leningrad
Uschakov PV (1955) Polychaeta of the Far Eastern Seas of the USSR. Keys to the fauna of the USSR 56:1–445
Uschakov PV (1959) Polychaeta. A list of the fauna of the sea waters of the South Sakhalin and South Kurile Islands. Explor Far East Seas USSR 6:201–208
Uschakov PV, Wu BL (1963) A preliminary zoogeographical studies on Polychaeta of the Yellow Sea. Oceanol Limnol Sin 5:154–164
Vaidya G, Lohman DJ, Meier R (2011) SequenceMatrix: concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 27:171–180
Wasson K, Zabin CJ, Bedinger L, Diaz MC, Pearse JS (2001) Biological invasions of estuaries without international shipping: the importance of intraregional transport. Biol Conser 102:143–153
Williams SL, Davidson IC, Pasari JR, Ashton GV, Carlton JT, Crafton RE, Fontana RE, Grosholz ED, Miller AW, Ruiz GM, Zabin CJ (2013) Managing multiple vectors for marine invasions in an increasingly connected world. Bioscience 63:952–966. https://doi.org/10.1525/bio.2013.63.12.8
Wu BL, Chen M (1980) Morphology, ecology, reproduction and larval development of Pseudopolydora paucibranchiata (Okuda). Acta Zool Sin 26:356–364
Xia X (2013) DAMBE5: a comprehensive software package for data analysis in molecular biology and evolution. Mol Biol Evol 30:1720–1728. https://doi.org/10.1093/molbev/mst064
Xia X, Xie Z, Salemi M, Chen L, Wang Y (2003) An index of substitution saturation and its application. Mol Phylogen Evol 26:1–7. https://doi.org/10.1016/s1055-7903(02)00326-3
Ye L, Tang B, Wu K, Su Y, Wang R, Yu Z, Wang J (2015) Mudworm Polydora lingshuiensis sp. n is a new species that inhabits both shell burrows and mudtubes. Zootaxa 3986:88–100. https://doi.org/10.11646/zootaxa.3986.1.4
Ye L, Yao T, Wu L, Lu J, Wang J (2019) Morphological and molecular diagnoses of Polydora brevipalpa Zachs, 1933 (Annelida: Spionidae) from the shellfish along the coast of China. J Oceanol Limnol 37:713–723. https://doi.org/10.1007/s00343-019-7381-0
Yokoyama H (1994) Influence of organic pollution on the macrobenthos in an urban estuary. Bull Natl Res Inst Aquac Suppl 1:1–8
Yokoyama H, Hayashi I (1980) Zonation and species diversity of smaller macrobenthos in the westernmost part of Wakasa Bay (the Sea of Tango). J Oceanogr Soc Jpn 36:46–58
Yoon SP, Kim YJ, Jung RH, Moon CH, Hong SJ, Lee WC, Park JS (2008) Benthic environments and macrobenthic polychaete community structure in the winter of 2005-2006 in Gamak Bay, Korea. Sea J Kor Soc Oceanogr 13:67–82
Zachs IG (1933) Polychaeta of the North Japan Sea. Explor Seas USSR 19:125–137
Zhou J, Ji WW, Li XZ (2010) Records of Polydora complex spionids (Polychaeta: Spionidae) from China’s coastal waters, with emphasis on parasitic species and the description of a new species. Mar Fish 32:1–15
Acknowledgements
Our sincere thanks to Robin S. Wilson (Australia), Christopher J. Glasby (Australia), Graham E. Gillespie (Canada), Céline Houbin (France), Hwey-Lian Hsieh (Taiwan), Jin-Woo Choi (South Korea), Marco Lezzi (Italy), Marco Faasse (Netherlands), and Temir A. Britayev (Russia) for providing material for molecular analysis or help in the field. Ertan Dağli (Turkey), Elianne P. Omena (Brazil) and Barbara Mikac (Croatia) provided specimens for morphological examination. Vyatcheslav V. Potin (Russia), Leslie H. Harris (USA), Christina Piotrowski (USA), Linda Ward (USA) and Xin-zheng Li (China) helped with museum collections. We thank Seungshic Yum for help with sequencing the material and James Blake for clarifying early records for some California localities. Vitaly O. Pois and Eugene Shvetsov helped with VIR database and the QGIS software. We thank two anonymous reviewers for their important comments. To all these colleagues, we express our sincere gratitude.
Funding
This study was funded by the Kuwait Institute for Scientific Research (KISR) and Kuwait Petroleum Corporation (KPC) for the Project “Biodiversity, distribution and abundance of intertidal macrofauna in Kuwait” (EM075C), Norwegian Research Council (Project 233635/H30 “Environmental management of petroleum activities in the Barents Sea: Norwegian-Russian collaboration”), and the Far Eastern Branch of the Russian Academy of Sciences (Grant 18-4-040).
Author information
Authors and Affiliations
Contributions
VIR formulated the idea, collected and examined most of the material, wrote the first draft of the manuscript. VVM and VVP sequenced material and analyzed molecular data. MCG, AG and EK collected and examined material in Italy. AN collected and sequenced material from Norway. MAK organised sampling in the Arabian Gulf and financial support for this study. JTC provided additional records, new insights on the biogeography and final editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Radashevsky, V.I., Malyar, V.V., Pankova, V.V. et al. Disentangling invasions in the sea: molecular analysis of a global polychaete species complex (Annelida: Spionidae: Pseudopolydora paucibranchiata). Biol Invasions 22, 3621–3644 (2020). https://doi.org/10.1007/s10530-020-02346-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-020-02346-x