Correction of Potassium Fertigation Rate of Apple Tree (Malus domestica Borkh.) in Central Russia during the Growing Season
Abstract
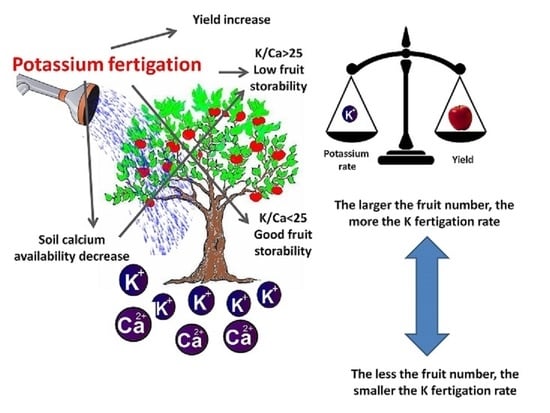
:1. Introduction
2. Materials and Methods
2.1. Research Location and Methods
2.2. Weather Conditions in 2016 and 2017
3. Results
3.1. Development of Some Productivity Components
3.2. Impact of Potassium Nutrition on the Amount of Yield
3.3. Seasonal Changes of Exchangeable Potassium and Calcium in Soil of the Experimental Plots
3.4. Seasonal Changes of Leaf Potassium Status and Its Relationship with Yield
3.5. Seasonal Changes of Leaf Calcium Status and Its Relationship with the Potassium Application Rate
3.6. The Content of Potassium in Apple Fruits and Its Relationship with Yield
3.7. The Content of Calcium in Apple Frits and Its Relationship with Potassium Application Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheng, L. Optimizing Nitrogen and Potassium Management to Foster Apple Tree Growth and Cropping Without Getting ‘Burned’. NYFQ 2013, 21, 21–24. Available online: http://nyshs.org/wp-content/uploads/2016/10/4.Optimizing-Nitrogen-and-Potassium-Management-to-Foster-Apple-Tree-Growth-and-Cropping-Without-Getting-Burned.pdf (accessed on 2 May 2020).
- Hou, W.; Tränkner, M.; Lu, J.; Yan, J.; Huang, S.; Ren, T.; Cong, R.; Li, X. Interactive effects of nitrogen and potassium on photosynthesis and photosynthetic nitrogen allocation of rice leaves. BMC Plant Biol. 2019, 19, 302. [Google Scholar] [CrossRef]
- Nieves-Cordones, M.; Al Shiblawi, F.; Sentenac, H. Roles and Transport of Sodium and Potassium in Plants. In The Alkali Metal Ions: Their Role for Life, 1st ed.; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Springer International Publishing AG: Cham, Switzerland, 2016; Volume 16, pp. 291–324. [Google Scholar] [CrossRef]
- Holb, I.J.; Conda, I.; Vágó, I. Seasonal Dynamics of Nitrogen, Phosphorus, and Potassium Contents of Leaf and Soil in Environmental Friendly apple Orchards. Commun. Soil Sci. Plant Anal. 2009, 40, 694–705. [Google Scholar] [CrossRef]
- Cheng, L.; Raba, R. Accumulation of Macro- and Micronutrients and Nitrogen Demand-supply Relationship of ‘Gala’/’Malling 26’ Apple Trees Grown in Sand Culture. J. Amer. Soc. Hort. Sci. 2009, 134, 3–13. [Google Scholar] [CrossRef]
- Hilmelrich, D.G.; Walker, S.E. Seasonal trends of calcium, magnesium, and potassium fractions in apple leaf and fruit tissues. J. Am. Soc. Hortic. Sci. 1982, 107, 1078–1080. [Google Scholar]
- Nachtigall, G.R.; Dechen, A.R. Seasonality of nutrients in leaves and fruits of apple trees. Sci. Agric. 2006, 63, 493–501. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.F.; Reuther, W.; Specht, A.W.; Hrnciar, G. Effect of Differential nitrogen, potassium and Magnesium Supply to Young Valencia Orange Trees in Sand culture on Mineral Composition Especially of Leaves and Fibrous Roots. Plant Physiol. 1954, 29, 349–355. [Google Scholar] [CrossRef] [Green Version]
- Kuzin, A.I.; Trunov, Y.V.; Solovyev, A.V.; Titova, L.V. Correction of leaf diagnosis parameters of phosphoric nutrition in different periods of apple vegetation in the Central Chernozem Region. Pomic. Small Fruits Cult. Russ. 2018, 52, 128–135. (In Russian) [Google Scholar] [CrossRef]
- Leszczyński, A.; Sadowski, A. Response of Apple Rootstocks and of Apple Maiden Trees to Different Levels of Potassium Supply in Sand Culture. Acta Hortic. 1990, 274, 277–286. [Google Scholar] [CrossRef]
- Xing, K.; Zhao, M.; Chen, C.; Wang, Y.; Xue, F.; Zhang, Y.; Dong, X.; Jiang, Y.; Chen, H.Y.H.; Kang, M. Leaf phosphorus content of Quercus wutaishanica increases with total soil potassium in the Loess Plateau. PLoS ONE 2018, 13, e0201350. [Google Scholar] [CrossRef]
- Szewczuk, A.; Komosa, A.; Gudarowska, E. Effect of soil potassium levels and different potassium fertilizer forms on yield and storability of ‘Golden delicious’ apples. Acta Sci. Pol. Hortorum Cultus. 2008, 7, 53–59. [Google Scholar]
- Kuzin, A.I.; Trunov, Y.V. Specific features of soil-leafy diagnostics for potassium nutrition of apple trees. Vestnik Russ. Agric. Sci. 2016, 1, 16–17. (In Russian) [Google Scholar]
- Roeva, T.A.; Leonicheva, E.V.; Leontieva, L.I. The impact of foliar fertilization on the phosphorus and potassium content in apple shoots. Pomic. Small Fruits Cult. Russ. 2018, 53, 183–188. (In Russian) [Google Scholar] [CrossRef]
- Tserling, V.V. Agricultural Crop Nutrition Diagnosis; Agropromizdat: Moscow, Soviet Union, 1990; pp. 165–166. (In Russian) [Google Scholar]
- Sanchez-Alonso, F.; Lachica, M. Oxalate salts in the leaves of plum (Prunus salcinica L.) and cherry (P. avium L.). New Phytol. 1988, 108, 505–508. [Google Scholar] [CrossRef]
- Lipa, T. Changes in chemical composition of leaves and shoots during vegetation of apple-tree rootstock in mother plantation. EJPAU 2013, 16, 8. Available online: http://www.ejpau.media.pl/volume16/issue2/art-08.html (accessed on 24 June 2020).
- Danner, M.A.; Scariotto, S.; Citadin, I.; Penso, G.A.; Cassol, L.C. Calcium sources applied to soil can replace leaf application in ‘Fuji’ apple tree. Pesq. Agropec. Trop. 2015, 45, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Dias, J.; Flore, J.A. Annual addition of potassium to the soil increased apple yield in Brazil. Commun. Soil Sci. Plant Anal. 2002, 33, 15–18. [Google Scholar] [CrossRef]
- Rather, G.H.; Bansal, S.K.; Bashir, O.; Weida, U. Impact of Potassium Nutrition on Fruit Yield and Physicochemical Characteristics of Apple Cultivar Red Delicious. Indian J. Fertil. 2019, 15, 790–797. Available online: https://www.ipipotash.org/uploads/abstracts/pdf/impact-potassium-nutrition-on-fruit-yield-and-characteristics-of-apple-ijf2019.pdf (accessed on 11 April 2020).
- Kuzin, A.I.; Trunov, Y.V.; Solovyev, A.V. Effect of fertigation on yield and fruit quality of apple (Malus domestica Borkh.) in high density orchards on chernozems in Central Russia. Acta Hortic. 2018, 1217, 343–350. [Google Scholar] [CrossRef]
- Mineev, V.G.; Sychev, V.G.; Amelyanchik, O.A.; Bolsheva, T.N.; Gomonova, N.F.; Durynina, E.P.; Egorov, V.S.; Egorova, E.V.; Edemskaya, N.L.; Karpova, E.A.; et al. Educational Aid on Agricultural Chemistry, 2nd ed.; Publishing House of Lomonosov Moscow State University: Moskow, Russia, 2001; pp. 90, 191, 410. (In Russian) [Google Scholar]
- Kuzin, A.I.; Trunov, Y.V.; Solovyev, A.V.; Smagin, B.I. Non-destructive diagnosis of apple tree potassium nutrition with reflective spectrophotometry. Pomic. Small Fruits Cult. Russ. 2018, 53, 147–156. (In Russian) [Google Scholar] [CrossRef]
- Tagliavini, M.; Scudellari, D.; Marangoni, B.; Bastianel, A.; Franzin, F.; Zamborlini, M. Leaf mineral composition of apple tree: Sampling date and effects of cultivar and rootstock. J. Plant Nutr. 1992, 15, 605–619. [Google Scholar] [CrossRef]
- Pacholak, E.; Zachwieja, M.; Zydlik, Z. Effect of nitrogen fertilization on the content of mineral components in soil, leaves and fruits of ‘Sampion’ apple trees. Acta Sci. Pol. Hortorum Cultus 2004, 3, 207–217. Available online: http://www.hortorumcultus.actapol.net/pub/3_2_207.pdf (accessed on 24 June 2020).
- Leonicheva, E.V.; Roeva, T.A.; Leonteva, L.I. Some features of calcium dynamics in the “apple fruit–leaves –shoots” system. Contemp. Hortic. 2018, 3, 131–138. (In Russian) [Google Scholar] [CrossRef]
- Zheng, W.; You, C.; Du, Z.; Zhai, H. Dynamic Changes in the Calcium Content of Several Apple Cultivars during the Growing Season. Agr. Sci. China. 2006, 5, 933–937. [Google Scholar] [CrossRef]
- Yoursuf, S.; Sheikh, M.A.; Chand, S.; Anjum, A. Effect of different sources of potassium on yield and quality of apple (cv. Red Delicious) in temperate conditions. J. Nat. Appl. Sci. 2019, 10, 1332–1340. [Google Scholar] [CrossRef] [Green Version]
- Zörb, C.; Senbauram, M.; Peiter, E. Potassium in agriculture–Status and perspectives. J. Plant. Nutr. 2014, 171, 656–669. [Google Scholar] [CrossRef]
- Zavalloni, C.; Marangoni, B.; Tagliavini, M.; Scudellari, D. Dynamics of Uptake of Calcium, Potassium and Magnesium into Apple Fruit in a High Density Planting. Acta Hortic. 2001, 564, 113–121. [Google Scholar] [CrossRef]
- Dar, M.A.; Wani, J.A.; Raina, S.K.; Bhat, M.Y.; Malik, M.A. Relationship of leaf nutrient content with fruit yield and quality of pear. J. Environ. Biol. 2015, 36, 649–653. Available online: http://www.jeb.co.in/journal_issues/201505_may15/paper_21.pdf (accessed on 12 July 2020).
- Zhang, W.; Zhang, X.; Wang, Y.; Zhang, N.; Guo, Y.; Ren, X.; Zhao, Z. Potassium fertilization arrests malate accumulation and alters soluble sugar metabolism in apple. Biol. Open 2018, 7, bio024745. [Google Scholar] [CrossRef] [Green Version]
- Tomala, K.; Trzak, M.; Koblińska, J. Prognosing Storage Ability of ‘Gloster’ Apples. Acta Hortic. 1994, 368, 578–585. [Google Scholar] [CrossRef]
- Marcelle, R.D. Mineral nutrition and fruit quality. Acta Hortic. 1995, 383, 219–226. [Google Scholar] [CrossRef]
- Kuzin, A.I. Influence of fertigation, drip irrigation and foliar nutrition on productivity of apple trees, fruit quality and soil properties in intensive orchard of the Central chernozem region. Sci. J. KubSAU 2017, 130, 1301706070. (In Russian) [Google Scholar] [CrossRef]
- Chen, Y.; Banin, A.; Borochovitch, A. Effect of potassium on soil structure in relation to hydraulic conductivity. Geoderma 1983, 30, 135–147. [Google Scholar] [CrossRef]
- RU 2593347. Available online: https://patentimages.storage.googleapis.com/c8/2f/14/53cd43c17750f1/RU2593347C1.pdf (accessed on 22 May 2020).
- Nielsen, D.; Nielsen, G. Nutritional effects on fruit quality for apple trees. NYFQ 2009, 17, 21–24. Available online: https://fruit.triforce.cals.wisc.edu/wp-content/uploads/sites/36/2016/03/Nutrition-effects-fruit-quality.pdf (accessed on 18 May 2020).
- Gudkovskii, V.A.; Kozhina, L.V.; Nazarov, Y.B.; Balakirev, A.E. Influence of pre-and post-harvest treatments with agrochemicals on the susceptibility of apple fruit to physiological and fungal diseases. In Scientific Foundations of Effective Horticulture: Proceedings of I.V. MichurIn Russiannn Research Institute for Horticulture (VNIIS im. I.V. Michurina); Kwarta: Voronezh, Russia, 2006; pp. 460–471. (In Russian) [Google Scholar]
- Dilmaghani, M.R.; Malakouti, M.J.; Neilsen, G.H.; Fallahi, E. Interactive Effects of Potassium and Calcium on K/Ca Ratio and its Consequences on Apple Fruit Quality in Calcareous Soils of Iran. J. Plant Nutr. 2005, 27, 1149–1162. [Google Scholar] [CrossRef]
- Wińska-Krysiak, M.; Łata, B. Influence of lipoxygenase activity and calcium and potassium contents on bitter pit occurrence in commercial apple cultivars. Folia Hortic. 2010, 22, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Stow, J. Effect of calcium ions on apple fruit softening during storage and ripening. Postharvest Biol. Tec. 1993, 3, 1–9. [Google Scholar] [CrossRef]
- Torres, E.; Recansens, I.; Lordan, J.; Alegre, S. Combination of strategies to supply calcium and reduce bitter pit in ‘Golden Delicious’ apples. Sci. Hortic. 2017, 217, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Solhjoo, S.; Gharaghani, A.; Fallahi, E. Calcium and Potassium Foliar Sprays Affect Fruit Skin Color, Quality Attributes, and Mineral Nutrient Concentrations of ‘Red Delicious’ Apples. Int. J. Fruit Sci. 2017, 17, 358–373. [Google Scholar] [CrossRef]
- Ernanini, P.R.; Dias, J.; Do Amarante, C.V.T.; Ribeiro, D.C.; Rogeri, D.A. Preharvest calcium sprays were not always needed to improve fruit quality of ‘Gala’ apples in Brazil. Rev. Bras. Frutic. 2008, 30, 892–896. Available online: https://www.scielo.br/pdf/rbf/v30n4/a09v30n4.pdf (accessed on 7 August 2020). [CrossRef] [Green Version]
- Saure, M.C. Calcium translocation to fleshy fruit: Its mechanism and endogenous control. Sci. Hortic. 2005, 105, 65–89. [Google Scholar] [CrossRef]






| Plot | Layer, cm | Fraction Sizes, mm | |||||
|---|---|---|---|---|---|---|---|
| >5 | 5–3 | 3–1 | 1–0.5 | 0.5–0.25 | <0.25 | ||
| % of the Studied Soil Sample | |||||||
| Cv. “Zhigulevskoye” | 0–30 | 3.53 | 0.29 | 5.76 | 37.53 | 13.96 | 38.93 |
| 30–60 | 0.00 | 0.14 | 7.45 | 35.78 | 15.24 | 41.39 | |
| Cv. “Lobo” | 0–30 | 2.17 | 0.36 | 2.92 | 27.63 | 20.77 | 46.15 |
| 30–60 | 0.00 | 0.00 | 2.95 | 33.66 | 19.80 | 43.59 | |
| G1, 2016, 2017 | G2, 2016 | G2, 2017 |
|---|---|---|
|
|
|
| Stages | 54;55 | 57;61;67 | 71; 72;74 | 76;78;81 |
|---|---|---|---|---|
| Sprayings | N, P, K, Mg, Fe, Mn, Zn, Cu, Mo, amino acid product | N, P, K, B, Mg, Fe, Mn, Zn, Cu, Mo | N, P, K, Mg, Fe, Mn, Zn, Cu, Mo | N, P, K, Mg, Fe, Mn, Zn, Cu, Mo |
| Month | Many Year Averages of Mean Air Temperature (1965–2015) | Year | |||
|---|---|---|---|---|---|
| 2016 | 2017 | ||||
| Temperature, °C | Variation | Temperature, °C | Variation | ||
| April | 6.8 | 9.5 | 2.7 | 7.2 | 0.4 |
| May | 14.5 | 14.8 | 0.3 | 13.3 | −1.2 |
| June | 18 | 18.8 | 0.8 | 18.3 | 0.3 |
| July | 19.4 | 21.9 | 2.5 | 19.4 | 0.0 |
| August | 18.1 | 21.3 | 3.2 | 20.4 | 2.3 |
| September | 12.3 | 11.7 | −0.6 | 14.0 | 1.7 |
| October | 5.1 | 5.1 | 0.1 | 5.5 | 0.4 |
| Mean IV–X | 13.5 | 14.7 | 1.2 | 14.0 | 0.5 |
| Month | Many Year Average Sums of Precipitation (1965–2015) | Year | |||
|---|---|---|---|---|---|
| 2016 | 2017 | ||||
| Precipitation, mm | % of Norm | Precipitation, mm | % of Norm | ||
| April | 37.1 | 104.6 | 281.9 | 28.2 | 76.0 |
| May | 52.4 | 126.7 | 241.8 | 27.9 | 53.2 |
| June | 55.7 | 83.5 | 149.9 | 30.9 | 55.5 |
| July | 69.9 | 56.7 | 81.1 | 106.2 | 151.9 |
| August | 60.0 | 78.1 | 130.2 | 74.9 | 124.8 |
| September | 55.5 | 39.0 | 70.3 | 20.0 | 36.0 |
| October | 46.2 | 24.8 | 53.7 | 47.2 | 102.2 |
| Mean IV–X | 53.8 | 73.3 | 136.2 | 47.9 | 159.2 |
| Group | Treatments | Number of Clusters | Number of Small Fruits (20–25 mm) | Number of Fruits (40–45 mm) |
|---|---|---|---|---|
| cv. “Zhigulevskoye” | ||||
| Control N20P15 | 189 | 101 | 83 | |
| G1 (unchangeable K rates) | N20P15K25 | 177 | 94 | 85 |
| N20P15K35 | 170 | 90 | 81 | |
| N20P15K45 | 181 | 96 | 86 | |
| G2 (season adjusted K rates) | N20P15K20 | 175 | 89 | 82 |
| N20P15K25 | 188 | 97 | 87 | |
| N20P15K30 | 194 | 104 | 91 | |
| LSD05 | 12 | 6 | 4 | |
| cv. “Lobo” | ||||
| Control N20P15 | 152 | 80 | 55 | |
| G1 (unchangeable K rates) | N20P15K25 | 146 | 75 | 53 |
| N20P15K35 | 140 | 70 | 51 | |
| N20P15K45 | 144 | 76 | 58 | |
| G2 (season adjusted K rates) | N20P15K20 | 136 | 68 | 52 |
| N20P15K25 | 143 | 72 | 55 | |
| N20P15K30 | 147 | 75 | 55 | |
| LSD05 | 9 | 4 | 4 | |
| Group | Treatments | Number of Clusters | Number of Small Fruits (20–25 mm) | Number of Fruits (40–45 mm) |
|---|---|---|---|---|
| cv. “Zhigulevskoye” | ||||
| Control N20P15 | 224 | 126 | 107 | |
| G1 (unchangeable K rates) | N20P15K25 | 221 | 120 | 105 |
| N20P15K35 | 216 | 127 | 115 | |
| N20P15K45 | 228 | 143 | 121 | |
| G2 (season adjusted K rates) | N20P15K30 | 224 | 113 | 104 |
| N20P15K40 | 218 | 122 | 110 | |
| N20P15K50 | 233 | 135 | 125 | |
| LSD05 | 22 | 13 | 9 | |
| cv. “Lobo” | ||||
| Control N20P15 | 212 | 108 | 75 | |
| G1 (unchangeable K rates) | N20P15K25 | 224 | 111 | 76 |
| N20P15K35 | 218 | 114 | 81 | |
| N20P15K45 | 215 | 119 | 89 | |
| G2 (season adjusted K rates) | N20P15K30 | 212 | 101 | 77 |
| N20P15K40 | 216 | 106 | 81 | |
| N20P15K50 | 202 | 118 | 91 | |
| LSD05 | 18 | 8 | 5 | |
| Group | Treatments | cv. “Zhigulevskoye” | cv. “Lobo” |
|---|---|---|---|
| 2016 | |||
| Control N20P15 | 26.3 | 19.3 | |
| G1 (unchangeable K rates) | N20P15K25 | 28.1 | 22.7 |
| N20P15K35 | 27.0 | 21.8 | |
| N20P15K45 | 29.1 | 22.1 | |
| G2 (season adjusted K rates) | N20P15K20 | 27.8 | 21.4 |
| N20P15K25 | 28.8 | 22.9 | |
| N20P15K30 | 28.6 | 24.6 | |
| LSD05 | 2.2 | 1.7 | |
| 2017 | |||
| Control N20P15 | 36.3 | 28.8 | |
| G1 (unchangeable K rates) | N20P15K25 | 38.1 | 35.8 |
| N20P15K35 | 41.9 | 38.9 | |
| N20P15K45 | 48.4 | 39.8 | |
| G2 (season adjusted K rates) | N20P15K30 | 44.7 | 40.8 |
| N20P15K40 | 43.2 | 41.1 | |
| N20P15K50 | 52.6 | 44.3 | |
| LSD05 | 3.0 | 3.2 | |
| Treatments | 30.05 | 30.06 | 28.07 | 30.08 | 29.09 |
|---|---|---|---|---|---|
| cv. “Zhigulevskoye” | |||||
| Control N20P15 | 22.2 | 24.2 | 24.4 | 23.5 | 22.2 |
| N20P15K25 | 20.5 | 26.4 | 24.4 | 24.3 | 23.0 |
| N20P15K35 | 21.5 | 21.9 | 24.0 | 20.9 | 20.7 |
| N20P15K45 | 21.0 | 24.6 | 29.4 | 22.5 | 23.0 |
| LSD05 | 2.1 | 1.9 | 2.8 | 2.2 | 1.7 |
| cv. “Lobo” | |||||
| Control N20P15 | 17.8 | 15.5 | 19.6 | 20.1 | 18.4 |
| N20P15K25 | 17.9 | 15.7 | 21.6 | 20.6 | 21.3 |
| N20P15K35 | 16.7 | 13.8 | 20.1 | 21.7 | 22.1 |
| N20P15K45 | 17.3 | 16.8 | 18.3 | 18.2 | 23.2 |
| LSD05 | 1.4 | 1.9 | 2.3 | 2.0 | 2.4 |
| Treatments | 30.05 | 30.06 | 27.07 | 30.08 | 28.09 |
|---|---|---|---|---|---|
| cv. “Zhigulevskoye” | |||||
| Control N20P15 | 19.6 | 18.8 | 19.1 | 18.2 | 18.8 |
| N20P15K25 | 18.7 | 18.6 | 17.2 | 18.2 | 18.2 |
| N20P15K35 | 19.4 | 18.2 | 17.5 | 18.4 | 17.9 |
| N20P15K45 | 18.2 | 17.9 | 17.0 | 19.3 | 17.3 |
| LSD05 | 1.8 | 1.6 | 2.0 | 1.5 | 1.5 |
| cv. “Lobo” | |||||
| Control N20P15 | 15.7 | 15.6 | 21.4 | 20.9 | 20.1 |
| N20P15K25 | 15.9 | 15.7 | 21.0 | 21.4 | 22.2 |
| N20P15K35 | 16.3 | 20.1 | 21.7 | 22.1 | 19.9 |
| N20P15K45 | 15.5 | 20.6 | 20.1 | 17.6 | 20.2 |
| LSD05 | 1.4 | 1.6 | 1.8 | 1.6 | 2.2 |
| Control N20P15 | N20P15K25 | N20P15K35 | N20P15K45 | N20P15K20 | N20P15K25 | N20P15K30 | LSD05 | |
|---|---|---|---|---|---|---|---|---|
| cv. “Zhigulevskoye” | ||||||||
| 30.05. | 1.81 | 1.89 | 1.74 | 1.97 | 1.76 | 1.92 | 1.73 | 0.10 |
| 30.06. | 1.61 | 1.21 | 1.29 | 1.56 | 1.28 | 1.21 | 1.26 | 0.08 |
| 28.07. | 1.36 | 1.09 | 1.32 | 1.33 | 1.33 | 1.29 | 1.24 | 0.08 |
| 30.08. | 1.22 | 1.05 | 1.00 | 0.98 | 0.94 | 0.88 | 0.98 | 0.06 |
| 29.09. | 1.30 | 1.27 | 1.31 | 1.52 | 1.12 | 1.37 | 1.44 | 0.08 |
| cv. “Lobo” | ||||||||
| 30.05. | 1.67 | 1.77 | 1.78 | 1.95 | 1.71 | 1.73 | 1.68 | 0.11 |
| 30.06. | 1.54 | 1.65 | 1.77 | 1.67 | 1.63 | 1.68 | 1.61 | 0.10 |
| 28.07. | 1.12 | 1.34 | 1.42 | 1.24 | 1.42 | 1.51 | 1.30 | 0.08 |
| 30.08. | 1.04 | 1.07 | 1.03 | 1.13 | 0.85 | 0.88 | 0.79 | 0.05 |
| 29.09. | 1.15 | 0.91 | 1.09 | 1.03 | 0.85 | 0.87 | 0.96 | 0.06 |
| Control N20P15 | N20P15K25 | N20P15K35 | N20P15K45 | N20P15K30 | N20P15K40 | N20P15K50 | LSD05 | |
|---|---|---|---|---|---|---|---|---|
| cv. “Zhigulevskoye” | ||||||||
| 30.05. | 1.88 | 1.84 | 1.96 | 2.18 | 1.81 | 1.93 | 1.88 | 0.08 |
| 30.06. | 1.52 | 1.20 | 1.23 | 1.30 | 1.23 | 1.34 | 1.38 | 0.07 |
| 27.07. | 1.52 | 1.18 | 1.21 | 1.42 | 1.15 | 1.12 | 1.29 | 0.07 |
| 30.08. | 0.86 | 0.90 | 0.88 | 0.79 | 0.83 | 0.79 | 0.76 | 0.06 |
| 28.09. | 1.35 | 1.02 | 1.10 | 1.33 | 1.15 | 1.11 | 1.23 | 0.06 |
| cv. “Lobo” | ||||||||
| 30.05. | 1.92 | 1.99 | 2.07 | 2.22 | 1.93 | 1.92 | 1.99 | 0.11 |
| 30.06. | 1.76 | 1.82 | 1.53 | 1.98 | 1.68 | 1.64 | 1.61 | 0.09 |
| 27.07. | 1.22 | 1.31 | 1.16 | 1.54 | 1.24 | 1.35 | 1.3 | 0.05 |
| 30.08. | 0.76 | 0.97 | 0.89 | 0.88 | 0.99 | 0.94 | 1.06 | 0.06 |
| 28.09. | 1.95 | 1.74 | 1.91 | 1.84 | 1.65 | 1.56 | 1.61 | 0.08 |
| Control N20P15 | N20P15K25 | N20P15K35 | N20P15K45 | N20P15K20 | N20P15K25 | N20P15K30 | LSD05 | |
|---|---|---|---|---|---|---|---|---|
| G1 (unchangeable K rates) | G2 (season adjusted K rates) | |||||||
| “Zhigulevskoye” | ||||||||
| 30.05. | 1.13 | 1.09 | 1.09 | 1.08 | 1.08 | 1.03 | 1.14 | 0.06 |
| 30.06. | 1.50 | 1.50 | 1.46 | 1.42 | 1.56 | 1.55 | 1.43 | 0.08 |
| 28.07. | 1.32 | 1.24 | 1.22 | 1.26 | 1.44 | 1.36 | 1.25 | 0.10 |
| 30.08. | 1.15 | 1.19 | 1.15 | 1.14 | 1.22 | 1.10 | 1.05 | 0.08 |
| 29.09. | 1.90 | 2.07 | 1.99 | 1.98 | 1.93 | 1.81 | 1.74 | 0.11 |
| “Lobo” | ||||||||
| 30.05. | 1.35 | 1.27 | 1.26 | 1.27 | 1.27 | 1.17 | 1.24 | 0.07 |
| 30.06. | 1.78 | 1.74 | 1.78 | 1.65 | 1.77 | 1.6 | 1.64 | 0.08 |
| 28.07. | 1.69 | 1.68 | 1.54 | 1.43 | 1.58 | 1.43 | 1.37 | 0.07 |
| 30.08. | 1.37 | 1.23 | 1.14 | 1.08 | 1.31 | 1.28 | 1.22 | 0.07 |
| 29.09. | 1.67 | 1.56 | 1.63 | 1.67 | 1.62 | 1.55 | 1.59 | 0.08 |
| Control N20P15K0 | N20P15K25 | N20P15K35 | N20P15K45 | N20P15K30 | N20P15K40 | N20P15K50 | HCP05 | |
|---|---|---|---|---|---|---|---|---|
| G1 (unchangeable K rates) | G2 (season adjusted K rates) | |||||||
| cv. “Zhigulevskoye” | ||||||||
| 30.05. | 1.34 | 1.36 | 1.32 | 1.36 | 1.35 | 1.34 | 1.30 | 0.07 |
| 30.06. | 1.70 | 1.36 | 1.48 | 1.44 | 1.38 | 1.34 | 1.24 | 0.08 |
| 27.07. | 1.57 | 1.65 | 1.55 | 1.24 | 1.27 | 1.26 | 1.22 | 0.08 |
| 30.08. | 1.28 | 1.20 | 1.18 | 1.24 | 1.19 | 1.17 | 1.26 | 0.08 |
| 28.09. | 1.59 | 1.59 | 1.71 | 1.52 | 1.65 | 1.66 | 1.54 | 0.09 |
| cv. “Lobo” | ||||||||
| 30.05. | 1.23 | 1.19 | 1.25 | 1.21 | 1.23 | 1.24 | 1.18 | 0.05 |
| 30.06. | 1.47 | 1.44 | 1.51 | 1.66 | 1.45 | 1.48 | 1.30 | 0.08 |
| 27.07. | 1.25 | 1.22 | 1.27 | 1.19 | 1.24 | 1.24 | 1.21 | 0.08 |
| 30.08. | 1.15 | 1.02 | 1.07 | 1.00 | 1.06 | 1.09 | 1.03 | 0.07 |
| 28.09. | 1.26 | 1.25 | 1.22 | 1.19 | 1.24 | 1.32 | 1.30 | 0.07 |
| Group | Treatments | cv. “Zhigulevskoye” | cv. “Lobo” |
|---|---|---|---|
| 2016 | |||
| Control N20P15 | 0.97 | 0.80 | |
| G1 (unchangeable K rates) | N20P15K25 | 1.12 | 0.86 |
| N20P15K35 | 1.04 | 0.82 | |
| N20P15K45 | 0.96 | 0.92 | |
| G2 (season adjusted K rates) | N20P15K20 | 0.84 | 0.73 |
| N20P15K25 | 0.87 | 0.73 | |
| N20P15K30 | 0.83 | 0.75 | |
| LSD05 | 0.07 | 0.06 | |
| 2017 | |||
| Control N20P15 | 0.58 | 0.73 | |
| G1 (unchangeable K rates) | N20P15K25 | 0.68 | 0.71 |
| N20P15K35 | 0.64 | 0.69 | |
| N20P15K45 | 0.61 | 0.75 | |
| G2 (season adjusted K rates) | N20P15K30 | 0.55 | 0.68 |
| N20P15K40 | 0.57 | 0.71 | |
| N20P15K50 | 0.62 | 0.78 | |
| LSD05 | 0.04 | 0.05 | |
| Group | Treatments | cv. “Zhigulevskoye” | cv. “Lobo” |
|---|---|---|---|
| 2016 | |||
| Control N20P15 | 0.043 | 0.050 | |
| G1 (unchangeable K rates) | N20P15K25 | 0.035 | 0.034 |
| N20P15K35 | 0.037 | 0.031 | |
| N20P15K45 | 0.038 | 0.033 | |
| G2 (season adjusted K rates) | N20P15K20 | 0.039 | 0.041 |
| N20P15K25 | 0.038 | 0.034 | |
| N20P15K30 | 0.036 | 0.037 | |
| LSD05 | 0.003 | 0.004 | |
| 2017 | |||
| Control N20P15 | 0.037 | 0.040 | |
| G1 (unchangeable K rates) | N20P15K25 | 0.024 | 0.033 |
| N20P15K35 | 0.021 | 0.035 | |
| N20P15K45 | 0.024 | 0.029 | |
| G2 (season adjusted K rates) | N20P15K30 | 0.028 | 0.034 |
| N20P15K40 | 0.028 | 0.031 | |
| N20P15K50 | 0.027 | 0.032 | |
| LSD05 | 0.004 | 0.004 | |
| Group | Treatments | cv. “Zhigulevskoye” | cv. “Lobo” |
|---|---|---|---|
| 2016 | |||
| Control N20P15 | 22.6 | 16.0 | |
| G1 (unchangeable K rates) | N20P15K25 | 31.1 | 25.3 |
| N20P15K35 | 28.1 | 26.5 | |
| N20P15K45 | 29.1 | 27.9 | |
| G2 (season adjusted K rates) | N20P15K20 | 23.3 | 17.8 |
| N20P15K25 | 22.9 | 21.5 | |
| N20P15K30 | 23.7 | 20.3 | |
| LSD05 | 1.7 | 1.3 | |
| 2017 | |||
| Control N20P15 | 14.9 | 18.3 | |
| G1 (unchangeable K rates) | N20P15K25 | 28.3 | 21.5 |
| N20P15K35 | 30.5 | 27.6 | |
| N20P15K45 | 25.4 | 25.9 | |
| G2 (season adjusted K rates) | N20P15K30 | 16.7 | 17.9 |
| N20P15K40 | 15.0 | 17.3 | |
| N20P15K50 | 16.3 | 22.8 | |
| LSD05 | 1.5 | 1.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuzin, A.I.; Kashirskaya, N.Y.; Kochkina, A.M.; Kushner, A.V. Correction of Potassium Fertigation Rate of Apple Tree (Malus domestica Borkh.) in Central Russia during the Growing Season. Plants 2020, 9, 1366. https://doi.org/10.3390/plants9101366
Kuzin AI, Kashirskaya NY, Kochkina AM, Kushner AV. Correction of Potassium Fertigation Rate of Apple Tree (Malus domestica Borkh.) in Central Russia during the Growing Season. Plants. 2020; 9(10):1366. https://doi.org/10.3390/plants9101366
Chicago/Turabian StyleKuzin, Andrei I., Natalia Ya. Kashirskaya, Anna M. Kochkina, and Alexey V. Kushner. 2020. "Correction of Potassium Fertigation Rate of Apple Tree (Malus domestica Borkh.) in Central Russia during the Growing Season" Plants 9, no. 10: 1366. https://doi.org/10.3390/plants9101366