Visual Adaptations in Predatory and Scavenging Diurnal Raptors
Abstract
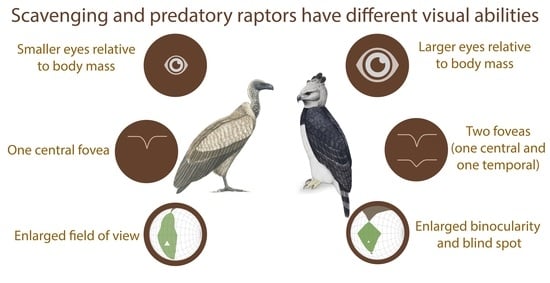
:1. Introduction
2. Anatomical Specialization of the Eye
2.1. Predators Have Larger Eyes Than Scavengers Relative to Their Body Mass
2.2. A Shared Optical System
2.3. Predators, but Not Scavengers, are Bifoveate
3. Foraging by Sight
3.1. The Spatial Resolution of Vision Does Not Depend on the Foraging Mode
3.2. Enlarged Field of View in Scavengers, Enlarged Binocularity in Predators
4. A Lack of Knowledge of Visual Abilities of Raptors, Especially of Scavengers
4.1. Contrast Sensitivity
4.2. Temporal Resolution of Vision
4.3. Colour Vision
5. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Stevens, M. Sensory Ecology, Behaviour, and Evolution; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Land, M.F.; Nilsson, D.-E. Animal Eyes; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Martin, G.R. The Sensory Ecology of Birds; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Mitkus, M.; Potier, S.; Martin, G.R.; Duriez, O.; Kelber, A. Raptor vision. In Oxford Research Encyclopedia of Neuroscience; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Potier, S.; Mitkus, M.; Kelber, A. Visual adaptations of diurnal and nocturnal raptors. Semin. Cell Dev. Biol. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Potier, S. Olfaction in raptors. Zool. J. Linn. Soc. 2019, 189, 713–721. [Google Scholar] [CrossRef]
- Reymond, L. Spatial visual acuity of the eagle Aquila audax: A behavioural, optical and anatomical investigation. Vis. Res. 1985, 25, 1477–1491. [Google Scholar] [CrossRef]
- Fischer, A.B. Laboruntersuchungen und freilandbeobachtungen zum sehvermögen und verhalten von altweltgeiern. Zool. Jahrb. Syst. Ökol. Geogr. Tiere 1968, 96, 81–132. [Google Scholar]
- Potier, S.; Mitkus, M.; Kelber, A. High resolution of colour vision, but low contrast sensitivity in a diurnal raptor. Proc. R. Soc. B Biol. Sci. 2018, 285, 20181036. [Google Scholar] [CrossRef] [Green Version]
- McClure, C.J.W.; Schulwitz, S.E.; Anderson, D.L.; Robinson, B.W.; Mojica, E.L.; Therrien, J.-F.; Oleyar, M.D.; Johnson, J. Commentary: Defining Raptors and Birds of Prey. J. Raptor Res. 2019, 53, 419–430. [Google Scholar] [CrossRef] [Green Version]
- Jetz, W.; Thomas, G.H.; Joy, J.B.; Hartmann, K.; Mooers, A.O. The global diversity of birds in space and time. Nature 2012, 491, 444–448. [Google Scholar] [CrossRef]
- Ferguson-Lees, J.; Christie, D.A. Raptors of the World; A&C Black: London, UK, 2001. [Google Scholar]
- Murn, C. Observations of predatory behavior by white-headed vultures. J. Raptor Res. 2014, 48, 297–299. [Google Scholar] [CrossRef]
- Kapfer, J.M.; Gammon, D.E.; Groves, J.D. Carrion-feeding by Barred Owls (Strix varia). Wilson J. Ornithol. 2011, 123, 646–649. [Google Scholar] [CrossRef]
- Milchev, B.; Spassov, N. First evidence for carrion—Feeding of Eurasian Eagle-owl (Bubo bubo) in Bulgaria. Ornis Hung. 2017, 25, 58–69. [Google Scholar] [CrossRef] [Green Version]
- Mori, E.; Menchetti, M.; Dartora, F. Evidence of carrion consumption behaviour in the Long-eared Owl Asio otus (Linnaeus, 1758) (Aves: Strigiformes: Strigidae). Ital. J. Zool. 2014, 81, 471–475. [Google Scholar] [CrossRef]
- Allen, M.L.; Taylor, A.P. First record of scavenging by a Western Screech-Owl (Megascops kennicottii). Wilson J. Ornithol. 2013, 125, 417–419. [Google Scholar] [CrossRef]
- Wilman, H.; Belmaker, J.; Simpson, J.; de la Rosa, C.; Rivadeneira, M.M.; Jetz, W. EltonTraits 1.0: Species-level foraging attributes of the world’s birds and mammals: Ecological Archives E095–179. Ecology 2014, 95, 2027. [Google Scholar] [CrossRef] [Green Version]
- Walls, G.L. The Vertebrate Eye and Its Adaptive Radiation; Cranbrook Institute of Science : Bloomfield Hills, MI, USA ; Cranbrook Press: Toowoomba City, QLD, Australia, 1942. [Google Scholar]
- Brooke, M.L.; Hanley, S.; Laughlin, S.B. The scaling of eye size with body mass in birds. Proc. R. Soc. B Biol. Sci. 1999, 266, 405–412. [Google Scholar] [CrossRef] [Green Version]
- Kiltie, R.A. Scaling of visual acuity with body size in mammals and birds. Funct. Ecol. 2000, 14, 226–234. [Google Scholar] [CrossRef]
- Niven, J.E.; Laughlin, S.B. Energy limitation as a selective pressure on the evolution of sensory systems. J. Exp. Biol. 2008, 211, 1792–1804. [Google Scholar] [CrossRef] [Green Version]
- Martin, G.R. Visual fields and their functions in birds. J. Ornithol. 2007, 148, 547–562. [Google Scholar] [CrossRef]
- Potier, S.; Mitkus, M.; Bonadonna, F.; Duriez, O.; Isard, P.-F.; Dulaurent, T.; Mentek, M.; Kelber, A. Eye size, fovea, and foraging ecology in accipitriform raptors. Brain Behav. Evol. 2017, 90, 232–242. [Google Scholar] [CrossRef]
- Zelenitsky, D.K.; Therrien, F.; Ridgely, R.C.; McGee, A.R.; Witmer, L.M. Evolution of olfaction in non-avian theropod dinosaurs and birds. Proc. R. Soc. B Biol. Sci. 2011, 279, 3625–3634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bang, B.G.; Cobb, S. The size of the olfactory bulb in 108 species of birds. Auk 1968, 85, 55–61. [Google Scholar]
- Corfield, J.R.; Price, K.; Iwaniuk, A.N.; Gutiérrez-Ibáñez, C.; Birkhead, T.; Wylie, D.R. Diversity in olfactory bulb size in birds reflects allometry, ecology, and phylogeny. Front. Neuroanat. 2015, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Tucker, V.A. The deep fovea, sideways vision and spiral flight paths in raptors. J. Exp. Biol. 2000, 203, 3745–3754. [Google Scholar] [PubMed]
- Potier, S.; Bonadonna, F.; Martin, G.R.; Isard, P.-F.; Dulaurent, T.; Mentek, M.; Duriez, O. Visual configuration of two species of Falconidae with different foraging ecologies. Ibis 2017, 160, 54–61. [Google Scholar] [CrossRef]
- Potier, S.; Duriez, O.; Cunningham, G.B.; Bonhomme, V.; O’Rourke, C.; Fernández-Juricic, E.; Bonadonna, F. Visual field shape and foraging ecology in diurnal raptors. J. Exp. Biol. 2018, 221, jeb177295. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis. Version 3.3.0; Springer-Verlag: New York, NY, USA, 2016. [Google Scholar]
- Ho, L.S.T.; Ane, C. phylolm: A linera-time algorithm for Gaussian and non-Gaussian trait evolution models. Syst. Biol. 2014, 63, 63–408. [Google Scholar]
- Revell, L.J. phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 2012, 3, 217–223. [Google Scholar] [CrossRef]
- Orme, D.; Freckleton, R.; Thomas, G.; Petzoldt, T.; Fritz, S.; Isaac, N.; Pearse, W. The caper: Comparative Analysis of Phylogenetics and Evolution in R. Available online: https://cran.r-project.org/web/packages/caper/vignettes/ (accessed on 24 September 2020).
- Zeileis, A.; Hothorn, T. lmtest: Diagnostic Checking in Regression Relationships. Available online: http://pkg.cs.ovgu.de/LNF/i386/5.10/R/LNFr-lmtest/reloc/R-2.10/library/lmtest/ (accessed on 24 September 2020).
- Kassambara, A. ggpubr: “ggplot2” based publication ready plots. R Package Version 0.2.5. 2020. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 24 September 2020).
- Wickham, H. plyr: The split-apply-combine strategy for data analysis. J. Stat. Soft. 2011, 40, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Dunning, J.B., Jr. CRC Handbook of Avian Body Masses; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Hwang, J.; Kang, S.; Seok, S.; Ahmed, S.; Yeon, S. Ophtalmic findings in cinereous vultures (Aegypius monachus). Vet. Ophthalmol. 2020, 23, 314–324. [Google Scholar] [CrossRef]
- Ritland, S.M. The Allometry of the Vertebrate Eye; Department of Biology, University of Chicago: Chicago, IL, USA, 1984. [Google Scholar]
- Spiegel, O.; Getz, W.M.; Nathan, R. Factors influencing foraging search efficiency: Why do scarce lappet-faced vultures outperform ubiquitous white-backed vultures? Am. Nat. 2013, 181, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.R. Schematic eye models in vertebrates. In Progress in Sensory Physiology; Springer: Berlin/Heidelberg, Germany, 1984; pp. 43–81. [Google Scholar]
- Miller, W.H. Ocular optical filtering. In Comparative Physiology and Evolution of Vision in Invertebrates; Springer: Berlin/Heidelberg, Germany, 1979; pp. 69–143. [Google Scholar]
- Glasser, A.; Howland, H.C. A history of studies of visual accommodation in birds. Q. Rev. Biol. 1996, 71, 475–509. [Google Scholar] [CrossRef] [PubMed]
- Ott, M. Visual accommodation in vertebrates: Mechanisms, physiological response and stimuli. J. Comp. Physiol. A 2006, 192, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Glasser, A.; Pardue, M.T.; Andison, M.E.; Sivak, J.G. A behavioral study of refraction, corneal curvature, and accommodation in raptor eyes. Can. J. Zool. 1997, 75, 2010–2020. [Google Scholar] [CrossRef] [Green Version]
- Sivak, J.G.; Hildebrand, T.; Lebert, C. Magnitude and rate of accommodation in diving and nondiving birds. Vis. Res. 1986, 25, 925–933. [Google Scholar] [CrossRef]
- Schaeffel, F.; Howland, H.C.; Farkas, L. Natural accommodation in the growing chicken. Vis. Res. 1986, 26, 1977–1993. [Google Scholar] [CrossRef]
- Rochon-Duvigneaud, A. Les yeux et la vision des vertébrés; Masson Paris: Paris, France, 1943. [Google Scholar]
- Fite, K.V.; Rosenfield-Wessels, S. A comparative study of deep avian foveas. Brain Behav. Evol. 1975, 12, 97–115. [Google Scholar] [CrossRef]
- Inzunza, O.; Bravo, H.; Smith, R.L.; Angel, M. Topography and morphology of retinal ganglion cells in Falconiforms: A study on predatory and carrion-eating birds. Anat. Rec. 1991, 229, 271–277. [Google Scholar] [CrossRef]
- Lisney, T.J.; Stecyk, K.; Kolominsky, J.; Graves, G.R.; Wylie, D.R.; Iwaniuk, A.N. Comparison of eye morphology and retinal topography in two species of new world vultures (Aves: Cathartidae). Anat. Rec. 2013, 296, 1954–1970. [Google Scholar] [CrossRef]
- Bringmann, A. Structure and function of the bird fovea. Anat. Histol. Embryol. 2019, 48, 177–200. [Google Scholar] [CrossRef]
- Oehme, H. Vergleichende untersuchungen an greifvogelaugen. Z. Für Morphol. Und Ökologie Der Tiere 1964, 53, 618–635. [Google Scholar] [CrossRef]
- Coimbra, J.P.; Collin, S.P.; Hart, N.S. Variations in retinal photoreceptor topography and the organization of the rod-free zone reflect behavioral diversity in Australian passerines. J. Comp. Neurol. 2015, 523, 1073–1094. [Google Scholar] [CrossRef]
- Bringmann, A.; Syrbe, S.; Görner, K.; Kacza, J.; Francke, M.; Wiedemann, P.; Reichenbach., A. The primate fovea: Structure, function and development. Prog. Retin. Eye Res. 2018, 66, 49–84. [Google Scholar] [CrossRef] [PubMed]
- Walls, G.L. Significance of the foveal depression. Arch. Ophthalmol. 1937, 18, 912–919. [Google Scholar] [CrossRef]
- Snyder, A.W.; Miller, W.H. Telephoto lens system of falconiform eyes. Nature 1979, 275, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Potier, S.; Mitkus, M.; Lisney, T.J.; Isard, P.-F.; Dulaurent, T.; Mentek, M.; Cornette, R.; Schikorski, D.; Kelber, A. Inter-individual differences in foveal shape in a scavenging raptor, the black kite Milvus migrans. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Reymond, L. Spatial visual acuity of the falcon, Falco berigora: A behavioural, optical and anatomical investigation. Vis. Res. 1987, 27, 1869–1874. [Google Scholar] [CrossRef]
- Pumphrey, P.J. The theory of the fovea. J. Exp. Biol. 1948, 25, 299–312. [Google Scholar]
- Ruggeri, M.; Major, J.C.; McKeown, C.; Knighton, R.W.; Puliafito, C.A.; Jiao, S. Retinal structure of birds of prey revealed by ultra-high resolution spectral-domain optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5789–5795. [Google Scholar] [CrossRef] [Green Version]
- Martin, G.R. What is binocular vision for? A birds’ eye view. J. Vis. 2009, 9, 14. [Google Scholar] [CrossRef] [Green Version]
- Moroney, M.K.; Pettigrew, J.D. Some observations on the visual optics of kingfishers (Aves, Coraciformes, Alcedinidae). J. Comp. Physiol. A 1987, 160, 137–149. [Google Scholar] [CrossRef]
- Tyrrell, L.P.; Fernández-Juricic, E. The hawk-eyed songbird: Retinal morphology, eye shape, and visual fields of an aerial insectivore. Am. Nat. 2017, 189, 709–717. [Google Scholar] [CrossRef] [Green Version]
- Fox, R.; Lehmkuhle, S.W.; Westendorf, D.H. Falcon visual acuity. Science 1976, 192, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, J. Falcon visual sensitivity to grating contrast. Nature 1982, 300, 57–58. [Google Scholar] [CrossRef]
- Potier, S.; Bonadonna, F.; Kelber, A.; Martin, G.R.; Isard, P.-F.; Dulaurent, T.; Duriez, O. Visual abilities in two raptors with different ecology. J. Exp. Biol. 2016, 219, 2639–2649. [Google Scholar] [CrossRef] [Green Version]
- Potier, S.; Bonadonna, F.; Kelber, A.; Duriez, O. Visual acuity in an opportunistic raptor, the chimango caracara (Milvago chimango). Physiol. Behav. 2016, 157, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Veilleux, C.C.; Kirk, E.C. Visual acuity in mammals: Effects of eye size and ecology. Brain Behav. Evol. 2014, 84, 43–53. [Google Scholar] [CrossRef]
- Martin, G.R.; Portugal, S.J. Differences in foraging ecology determine variation in visual fields in ibises and spoonbills (Threskiornithidae). Ibis 2011, 153, 662–671. [Google Scholar] [CrossRef]
- Martin, G.R.M.; Jarrett, N.; Williams, M. Visual fields in Blue Ducks Hymenolaimus malacorhynchos and Pink-eared Ducks Malacorhynchus membranaceus: Visual and tactile foraging. Ibis 2007, 149, 112–120. [Google Scholar] [CrossRef]
- Martin, G.R.; Piersma, T. Vision and touch in relation to foraging and predator detection: Insightful contrasts between a plover and a sandpiper. Proc. R. Soc. B Biol. Sci. 2009, 276, 437–445. [Google Scholar] [CrossRef]
- Boal, C. Golden Eagle predation of an adult Turkey Vulture. Bull. Tex. Ornithol. Soc. 2015, 48, 53–55. [Google Scholar]
- Thompson, L.J.; Clemence, L.; Clemence, B.; Goosen, D. Nestling White-backed Vulture (Gyps africanus) eaten by a Verreaux’s Eagle (Aquila verreauxii) at a nest occupied for a record 21 years. Vulture News 2018, 74, 24–30. [Google Scholar] [CrossRef]
- Fernández-Juricic, E.; Erichsen, J.T.; Kacelnik, A. Visual perception and social foraging in birds. Trends Ecol. Evol. 2004, 19, 25–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slagsvold, T.; Sonerud, G.A.; Grønlien, H.E.; Stige, L.C. Prey handling in raptors in relation to their morphology and feeding niches. J. Avian Biol. 2010, 41, 488–497. [Google Scholar] [CrossRef]
- Martin, G.R.; Coetzee, H.C. Visual fields in hornbills: Precision-grasping and sunshades. Ibis 2004, 146, 18–26. [Google Scholar] [CrossRef]
- Martin, G.R.; Katzir, G. Sun shades and eye size in birds. Brain Behav. Evol. 2000, 56, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Portugal, S.J.; Murn, C.P.; Martin, G.R. White-headed Vulture Trigonoceps occipitalis shows visual field characteristics of hunting raptors. Ibis 2017, 159, 463–466. [Google Scholar] [CrossRef]
- Martin, G.R.; Portugal, S.J.; Murn, C.P. Visual fields, foraging and collision vulnerability in Gyps vultures. Ibis 2012, 154, 626–631. [Google Scholar] [CrossRef]
- McClure, C.J.W.; Westrip, J.R.S.; Johnson, J.A.; Schulwitz, S.E.; Virani, M.Z.; Davies, R.; Symes, A.; Wheatley, H.; Thorstrom, R.; Amar, A. State of the world’s raptors: Distributions, threats, and conservation recommendations. Biol. Conserv. 2018, 227, 390–402. [Google Scholar] [CrossRef]
- Reymond, L.; Wolfe, J. Behavioural determination of the contrast sensitivity function of the eagle Aquila audax. Vis. Res. 1981, 21, 263–271. [Google Scholar] [CrossRef]
- Ghim, M.M.; Hodos, W. Spatial contrast sensitivity of birds. J. Comp. Physiol. A 2006, 192, 523–534. [Google Scholar] [CrossRef] [Green Version]
- Campbell, F.W.; Maffei, M.; Piccolino, M. The contrast sensitivity of the cat. J. Physiol. 1973, 229, 719–731. [Google Scholar] [CrossRef] [Green Version]
- Virsu, V.; Rovamo, J.; Laurinen, P.; Näsänen, R. Temporal contrast sensitivity and cortical magnification. Vis. Res. 1982, 22, 1211–1217. [Google Scholar] [CrossRef]
- Kane, A.; Jackson, A.L.; Ogada, D.L.; Monadjem, A.; McNally, L. Vultures acquire information on carcass location from scavenging eagles. Proc. R. Soc. B Biol. Sci. 2014, 281, 20141072. [Google Scholar] [CrossRef] [Green Version]
- Cortés-Avizanda, A.; Jovani, R.; Donázar, J.A.; Grimm, V. Bird sky networks: How do avian scavengers use social information to find carrion? Ecology 2014, 95, 1799–1808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, A.L.; Ruxton, G.D.; Houston, D.C. The effect of social facilitation on foraging success in vultures: A modelling study. Biol. Lett. 2008, 4, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Potier, S.; Lieuvin, M.; Pfaff, M.; Kelber, A. How fast can raptors see? J. Exp. Biol. 2020, 223, jeb209031. [Google Scholar] [CrossRef] [PubMed]
- Boström, J.E.; Dimitrova, M.; Canton, C.; Håstad, O.; Qvarnström, A.; Ödeen, A. Ultra-rapid vision in birds. PLoS ONE 2016, 11, e0151099. [Google Scholar] [CrossRef] [Green Version]
- Nuboer, J.F.W.; Coemans, M.; Vos, J.J. Artificial lighting in poultry houses: Do hens perceive the modulation of fluorescent lamps as flicker? Br. Poult. Sci. 1992, 33, 123–133. [Google Scholar] [CrossRef]
- Tucker, V.A. Gliding flight: Speed and acceleration of ideal falcons during diving and pull out. J. Exp. Biol. 1998, 201, 403–414. [Google Scholar]
- Srinivasan, M.V.; Bernard, G.D. The effect of motion on visual acuity of the compound eye: A theoretical analysis. Vis. Res. 1975, 15, 515–525. [Google Scholar] [CrossRef]
- Kelber, A. Bird colour vision—From cones to perception. Curr. Opin. Behav. Sci. 2019, 30, 34–40. [Google Scholar] [CrossRef]
- Hart, N.S.; Hunt, D.M. Avian visual pigments: Characteristics, spectral tuning, and evolution. Am. Nat. 2007, 169, S7–S26. [Google Scholar] [CrossRef]
- Ödeen, A.; Håstad, O. The phylogenetic distribution of ultraviolet sensitivity in birds. BMC Evol. Biol. 2013, 13, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lind, O.; Mitkus, M.; Olsson, P.; Kelber, A. Ultraviolet sensitivity and colour vision in raptor foraging. J. Exp. Biol. 2013, 216, 1819–1826. [Google Scholar] [CrossRef] [Green Version]
- Viitala, J.; Korpimäki, E.; Palokangas, P.; Koivula, M. Attraction of kestrels to vole scent marks visible in ultraviolet light. Nature 1995, 373, 425–427. [Google Scholar] [CrossRef]
- Koivula, M.; Viitala, J.; Koipimaki, E. Kestrels prefer scent marks according to species and reproductive status of voles. Ecoscience 1999, 6, 415–420. [Google Scholar] [CrossRef]
- Wu, Y.; Hadly, E.A.; Teng, W.; Hao, Y.; Liang, W.; Liu, Y.; Wang, H. Retinal transcriptome sequencing sheds light on the adaptation to nocturnal and diurnal lifestyles in raptors. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lind, O.; Kelber, A. The spatial tuning of achromatic and chromatic vision in budgerigars. J. Vis. 2011, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Dominoni, D.M.; Halfwerk, W.; Baird, E.; Buxton, R.T.; Fernández-Juricic, E.; Fristrup, K.M.; McKenna, M.F.; Mennitt, D.J.; Perkin, E.K.; Seymoure, B.M.; et al. Why conservation biology can benefit from sensory ecology. Nat. Ecol. Evol. 2020, 4, 502–511. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potier, S. Visual Adaptations in Predatory and Scavenging Diurnal Raptors. Diversity 2020, 12, 400. https://doi.org/10.3390/d12100400
Potier S. Visual Adaptations in Predatory and Scavenging Diurnal Raptors. Diversity. 2020; 12(10):400. https://doi.org/10.3390/d12100400
Chicago/Turabian StylePotier, Simon. 2020. "Visual Adaptations in Predatory and Scavenging Diurnal Raptors" Diversity 12, no. 10: 400. https://doi.org/10.3390/d12100400