Abstract
A novel series of quinolone-based heterocyclic derivatives including thiadiazine, thiadiazoles, and triazole were synthesized and their in vitro antibacterial activity against Gram-positive and Gram-negative bacteria were evaluated. Newly synthesized derivatives have been obtained in good yields ranging from 65 to 80%. The synthesized derivatives have been characterized and their structures identified using spectroscopic analysis including NMR, FT-IR, and mass techniques. Most of compounds exhibited moderate-to-good antibacterial activity against all four bacterial strains and are significantly more active than ampicillin. Compounds showed relatively good anti-bacterial activity compared to moderate activity of other compounds. The results obtained herein are important for further structure modifications of quinoline bearing heterocyclic moiety and the exploitation of the therapeutic potential of quinoline derivatives as antibacterial agents.
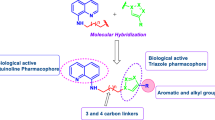
Graphic abstract

Similar content being viewed by others
References
Zinad DS, Mahal A, Mohapatra RK, Sarangi AK, Pratama MRF (2020) Chem Biol Drug Des 95:16
Zinad DS, Mahal A, Shareef OA (2020) IOP Conf Ser Mater Sci Eng 770:012053
Zinad DS, Shareef OA, Mahal A (2020) AIP Conf Proc 2213:020188
Mahal A, Wu P, Jiang Z-H, Wei X (2017) Nat Prod Bioprospect 7:461
Mahal A, Wu P, Jiang Z-H, Wei X (2019) ChemistrySelect 4:366
Batista VF, Pinto DCGA, Silva AMS (2016) ACS Sustain Chem Eng 4:4064
Quan X-J, Ren Z-H, Wang Y-Y, Guan Z-H (2014) Org Lett 16:5728
Niu P, Kang J, Tian X, Song L, Liu H, Wu J, Yu W, Chang J (2015) J Org Chem 80:1018
Terrab L, Rosenker CJ, Johnstone L, Ngo LK, Zhang L, Ware NF, Miller B, Topacio AZ, Sannino S, Brodsky JL, Wipf P (2020) ACS Med Chem Lett 11:984
Pretorius SI, Breytenbach WJ, de Kock C, Smith PJ (2013) Bioorg Med Chem 21:269
Mahamoud A, Chevalier J, Davin-Regli A, Barbe J, Pagès J-M (2006) Curr Drug Targets 7:843
Lilienkampf A, Mao J, Wan B, Wang Y, Franzblau SG, Kozikowski AP (2009) J Med Chem 52:2109
Yeh-Long C, Hsien-Ming H, Chih-Ming L, Kuang-Chieh L, Cherng-Chyi T (2004) Bioorg Med Chem 12:6539
Maguire MP, Sheets KR, McVety K, Spada AP, Zilberstein A (1994) J Med Chem 37:2129
Kumar GVS, Rajendraprasad Y, Mallikarjuna BP, Chandrashekar SM, Kistayya C (2010) Eur J Med Chem 45:2063
Guzeldemirci NU, Kucukbasmaci O (2010) J Med Chem 45:63
Karabasanagouda T, Adhikari AV, Shetty NS (2007) Eur J Med Chem 42:521
Mathew V, Keshavayya J, Vaidya VP (2006) Eur J Med Chem 41:1048
Bektas H, Demirbas A, Demirbas N, Karaoglu SA (2010) Turk J Chem 34:165
Bayrak H, Demirbas A, Demirbas N, Karaoglu SA (2009) Eur J Med Chem 44:4362
Jain AK, Sharma S, Vaidya A, Ravichandran V, Agrawal RK (2013) Chem Biol Drug Des 81:557
El-Shehry MF, Abu-Hashem AA, El-Telbani EM (2010) Eur J Med Chem 45:1906
Ammar YA, Ghorab MM, El-Sharief AMS, Mohamed SI (2002) Heteroat Chem 13:199
Pomarnacka E, Gdaniec M (2003) Bioorg Med Chem 11:1259
Hosam S (1996) Indian J Chem 35B:980
Thomas KD, Adhikari AV, Suchetha NS (2010) Eur J Med Chem 45:3803
Brischoff CA (1900) Ber Dtsch Chem Ges 33:924
Mahal A, Abu-El-Halawa R, Zabin SA, Ibrahim M, Al-Refai M, Kaimari T (2015) World J Org Chem 3:1
Boraei ATA, Ghabbour HA, Gomaa MS, El Ashry E-SH, Barakat A (2019) Molecules 24:4471
Fairbrother RW, Martyn G (1951) J Clin Pathol 4:374
Gould JC, Bowie JH (1952) Edinburgh Med J 59:178
Acknowledgements
Ghazwan Salman would like to express his sincere gratitude to Mustansiriyah University for providing financial support for one sabbatical year. Dhafer S. Zinad would like to express his great thanks to the University of Technology for providing facilities and support. Ahmed Mahal would like to acknowledge financial support through the CAS President’s International Fellowship Initiative (2016PM032), Chinese Academy of Sciences (CAS).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Salman, G.A., Zinad, D.S. & Mahal, A. Design, synthesis, and biological evaluation of new quinoline-based heterocyclic derivatives as novel antibacterial agents . Monatsh Chem 151, 1621–1628 (2020). https://doi.org/10.1007/s00706-020-02686-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-020-02686-3