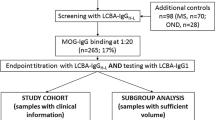
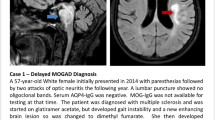
Abstract
Myelin oligodendrocyte glycoprotein (MOG) is a unique CNS-specific mammalian protein that is expressed on the surface of compact myelin and oligodendrocyte cell bodies. MOG is an accessible target for autoantibodies, associated with immune-mediated demyelination in the central nervous system. The identification of MOG reactive immunoglobulin G antibodies (MOG-IgG) helps to distinguish a subgroup of patients from multiple sclerosis and other CNS disorders, reducing the risk of clinical misdiagnosis. The development of the cell-based assays (CBA) improved the detection of clinically meaningful MOG-IgG binding to conformational MOG expressed in the cell membrane surface. In this review, we describe factors that impact on the results of CBA, such as MOG conformation, protein glycosylation, addition of fluorescent tags, serum dilution, secondary antibodies, and data interpretation.

Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article.
Abbreviations
- ADEM:
-
acute disseminated encephalomyelitis
- AQP4:
-
aquaporin-4
- CBA:
-
cell-based assay
- CHO:
-
Chinese hamster ovary
- CNS:
-
central nervous system
- eGFP:
-
enhanced green fluorescent protein
- ELISA:
-
enzyme-linked immunonosorbent assay
- FACS:
-
fluorescence-activated cell sorting
- FITC:
-
fluorescein isothiocyanate
- FL-MOG:
-
full-length MOG
- GFP:
-
green fluorescent protein
- HEK293:
-
human embryonic kidney 293
- IIF:
-
indirect immunofluorescence
- IgG:
-
immunoglobulin G
- IgM:
-
immunoglobulin M
- LGI1:
-
leucine-rich glioma inactivated 1
- MFI:
-
median fluorescent intensity
- MHC:
-
major histocompatibility complex
- MOG:
-
myelin oligodendrocyte glycoprotein
- mRNA:
-
messenger ribonucleic acid
- NMOSD:
-
neuromyelitis optica spectrum disorder
- PEI:
-
polyethylenimine
- RIA:
-
radioimmunoassay
- SL-MOG:
-
short-length MOG
- WB:
-
western blot
References
Ramanathan S, Dale RC, Brilot F (2016) Anti-MOG antibody: the history, clinical phenotype, and pathogenicity of a serum biomarker for demyelination. Autoimmun Rev 15:307–324. https://doi.org/10.1016/j.autrev.2015.12.004
Narayan R, Simpson A, Fritsche K, Salama S, Pardo S, Mealy M, Paul F, Levy M (2018) MOG antibody disease: a review of MOG antibody seropositive neuromyelitis optica spectrum disorder. Mult Scler Relat Disord 25:66–72. https://doi.org/10.1016/j.msard.2018.07.025
Asseyer S, Schmidt F, Chien C, Scheel M, Ruprecht K, Bellmann-Strobl J, Brandt AU, Paul F (2018) Pain in AQP4-IgG-positive and MOG-IgG-positive neuromyelitis optica spectrum disorders. Mult Scler J Exp Transl Clin 4:1–12. https://doi.org/10.1177/2055217318796684
Jarius S, Ruprecht K, Stellmann JP, Huss A, Ayzenberg I, Willing A, Trebst C, Pawlitzki M, Abdelhak A, Grüter T, Leypoldt F, Haas J, Kleiter I, Tumani H, Fechner K, Reindl M, Paul F, Wildemann B (2018) MOG-IgG in primary and secondary chronic progressive multiple sclerosis: a multicenter study of 200 patients and review of the literature. J Neuroinflammation 15:88. https://doi.org/10.1186/s12974-018-1108-6
O’Connor KC, McLaughlin KA, De Jager PL, Chitnis T, Bettelli E, Xu C et al (2007) Self-antigen tetramers discriminate between myelin autoantibodies to native or denatured protein. Nat Med 13:211–217. https://doi.org/10.1038/nm1488
dos Passos GR, Oliveira LM, da Costa BK, Apostolos-Pereira SL, Callegaro D, Fujihara K, Sato DK (2018) MOG-IgG-associated optic neuritis, encephalitis, and myelitis: lessons learned from neuromyelitis optica spectrum disorder. Front Neurol 9:1–10. https://doi.org/10.3389/fneur.2018.00217
Rosenthal JF, Hoffman BM, Tyor WR (2020) CNS inflammatory demyelinating disorders: MS, NMOSD and MOG antibody associated disease. J Investig Med 68:321–330. https://doi.org/10.1136/jim-2019-001126
Ogawa R, Nakashima I, Takahashi T, Kaneko K, Akaishi T, Takai Y, Sato DK, Nishiyama S, Misu T, Kuroda H, Aoki M, Fujihara K (2017) MOG antibody-positive, benign, unilateral, cerebral cortical encephalitis with epilepsy. Neurol Neuroimmunol NeuroInflammation 4:1–10. https://doi.org/10.1212/NXI.0000000000000322
Hamid SHM, Whittam D, Saviour M, Alorainy A, Mutch K, Linaker S, Solomon T, Bhojak M, Woodhall M, Waters P, Appleton R, Duddy M, Jacob A (2018) Seizures and encephalitis in myelin oligodendrocyte glycoprotein IgG disease vs aquaporin 4 IgG disease. JAMA Neurol 75:65–71. https://doi.org/10.1001/jamaneurol.2017.3196
Musso G, Nosadini M, Gallo N, Sartori S, Seguso M, Plebani M (2020) Possible clinical role of MOG antibody testing in children presenting with acute neurological symptoms. Neurol Sci 41:2553–2559. https://doi.org/10.1007/s10072-020-04379-5
Jarius S, Paul F, Aktas O, Asgari N, Dale RC, de Seze J, Franciotta D, Fujihara K, Jacob A, Kim HJ, Kleiter I, Kümpfel T, Levy M, Palace J, Ruprecht K, Saiz A, Trebst C, Weinshenker BG, Wildemann B (2018) MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J Neuroinflammation 15:1–10. https://doi.org/10.1007/s00115-018-0607-0
Reindl M, Waters P (2019) Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nat Rev Neurol 15:89–102. https://doi.org/10.1038/s41582-018-0112-x
Hacohen Y, Banwell B (2019) Treatment approaches for MOG-Ab-associated demyelination in children. Curr Treat Options Neurol 21:2. https://doi.org/10.1007/s11940-019-0541-x
Fujihara K, Sato DK, Nakashima I, Takahashi T, Kaneko K, Ogawa R, Akaishi T, Matsumoto Y, Takai Y, Nishiyama S, Kuroda H, Misu T, Aoki M (2018) Myelin oligodendrocyte glycoprotein immunoglobulin G-associated disease: an overview. Clin Exp Neuroimmunol. 9:48–55. https://doi.org/10.1111/cen3.12434
Johns TG, Bernard CCA (1999) The structure and function of myelin oligodendrocyte glycoprotein. J Neurochem 72:1–9. https://doi.org/10.1046/j.1471-4159.1999.0720001.x
Pham-Dinh D, Mattei MG, Nussbaum JL, Roussel G, Pontarotti P, Roeckel N, Mather IH, Artzt K, Lindahl KF, Dautigny A (1993) Myelin/oligodendrocyte glycoprotein is a member of a subset of the immunoglobulin superfamily encoded within the major histocompatibility complex. Proc Natl Acad Sci 90:7990–7994. https://doi.org/10.1073/pnas.90.17.7990
Allamargot C, Gardinier MV (2007) Alternative isoforms of myelin/oligodendrocyte glycoprotein with variable cytoplasmic domains are expressed in human brain. J Neurochem 101:298–312. https://doi.org/10.1111/j.1471-4159.2006.04296.x
Boyle LH, Traherne JA, Plotnek G, Ward R, Trowsdale J (2007) Splice variation in the cytoplasmic domains of myelin oligodendrocyte glycoprotein affects its cellular localisation and transport. J Neurochem 102:1853–1862. https://doi.org/10.1111/j.1471-4159.2007.04687.x
della Gaspera B, Pham-Dinh D, Roussel G, Nussbaum J-L, Dautigny A (1998) Membrane topology of the myelin/oligodendrocyte glycoprotein. Eur J Biochem 258:478–484. https://doi.org/10.1046/j.1432-1327.1998.2580478.x
Scolding NJ, Frith S, Linington C, Morgan BP, Campbell AK, Compston DAS (1989) Myelin-oligodendrocyte glycoprotein (MOG) is a surface marker of oligodendrocyte maturation. J Neuroimmunol 22:169–176. https://doi.org/10.1016/0165-5728(89)90014-3
Burger D, Steck AJ, Bernard CCA, de Rosbo NK (1993) Human myelin/oligodendrocyte glycoprotein: a new member of the L2/HNK-1 family. J Neurochem 61:1822–1827. https://doi.org/10.1111/j.1471-4159.1993.tb09822.x
Dyer CA, Matthieu J-M (1994) Antibodies to myelin/oligodendrocyte-specific protein and myelin/oligodendrocyte glycoprotein signal distinct changes in the Organization of Cultured Oligodendroglial Membrane Sheets. J Neurochem 62:777–787. https://doi.org/10.1046/j.1471-4159.1994.62020777.x
Johns TG, Bernard CCA (1997) Binding of complement component Clq to myelin oligodendrocyte glycoprotein: a novel mechanism for regulating CNS inflammation. Mol Immunol 34:33–38. https://doi.org/10.1016/S0161-5890(97)00005-9
von Büdingen HC, Mei F, Greenfield A, Jahn S, Shen YAA, Reid HH, McKemy DD, Chan JR (2015) The myelin oligodendrocyte glycoprotein directly binds nerve growth factor to modulate central axon circuitry. J Cell Biol 210:891–898. https://doi.org/10.1083/jcb.201504106
Reindl M, Linington C, Brehm U, Egg R, Dilitz E, Deisenhammer F, Poewe W, Berger T (1999) Antibodies against the myelin oligodendrocyte glycoprotein and the myelin basic protein in multiple sclerosis and other neurological diseases: a comparative study. Brain. 122:2047–2056. https://doi.org/10.1093/brain/122.11.2047
Berger T, Rubner P, Schautzer F, Egg R, Ulmer H, Mayringer I, Dilitz E, Deisenhammer F, Reindl M (2003) Antimyelin antibodies as a predictor of clinically definite multiple sclerosis after a first demyelinating event. N Engl J Med 349:139–145. https://doi.org/10.1056/NEJMoa022328
Egg R, Reindl M, Deisenhammer F, Linington C, Berger T (2001) Anti-MOG and anti-MBP antibody subclasses in multiple sclerosis. Mult Scler 7:285–289. https://doi.org/10.1191/135245801681137979
Di Pauli F, Mader S, Rostasy K, Schanda K, Bajer-Kornek B, Ehling R et al (2011) Temporal dynamics of anti-MOG antibodies in CNS demyelinating diseases. Clin Immunol 138:247–254. https://doi.org/10.1016/j.clim.2010.11.013
Brilot F, Dale RC, Selter RC, Grummel V, Kalluri SR, Aslam M et al (2009) Antibodies to native myelin oligodendrocyte glycoprotein in children with inflammatory demyelinating central nervous system disease. Ann Neurol 66:833–842. https://doi.org/10.1002/ana.21916
Lalive PH, Häusler MG, Maurey H, Mikaeloff Y, Tardieu M, Wiendl H, Schroeter M, Hartung HP, Kieseier BC, Menge T (2011) Highly reactive anti-myelin oligodendrocyte glycoprotein antibodies differentiate demyelinating diseases from viral encephalitis in children. Mult Scler J 17:297–302. https://doi.org/10.1177/1352458510389220
Mader S, Gredler V, Schanda K, Rostasy K, Dujmovic I, Pfaller K, Lutterotti A, Jarius S, di Pauli F, Kuenz B, Ehling R, Hegen H, Deisenhammer F, Aboul-Enein F, Storch MK, Koson P, Drulovic J, Kristoferitsch W, Berger T, Reindl M (2011) Complement activating antibodies to myelin oligodendrocyte glycoprotein in neuromyelitis optica and related disorders. J Neuroinflammation 8:1–14. https://doi.org/10.1186/1742-2094-8-184
Rostásy K, Mader S, Hennes EM, Schanda K, Gredler V, Guenther A, Blaschek A, Korenke C, Pritsch M, Pohl D, Maier O, Kuchukhidze G, Brunner-Krainz M, Berger T, Reindl M (2013) Persisting myelin oligodendrocyte glycoprotein antibodies in aquaporin-4 antibody negative pediatric neuromyelitis optica. Mult Scler J 19:1052–1059. https://doi.org/10.1177/1352458512470310
Kitley J, Woodhall M, Waters P, Leite MI, Devenney E, Craig J, Palace J, Vincent A (2012) Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology. 79:1273–1277. https://doi.org/10.1212/WNL.0b013e31826aac4e
Rostasy K, Mader S, Schanda K, Huppke P, Gärtner J, Kraus V, Karenfort M, Tibussek D, Blaschek A, Bajer-Kornek B, Leitz S, Schimmel M, di Pauli F, Berger T, Reindl M (2012) Anti–myelin oligodendrocyte glycoprotein antibodies in pediatric patients with optic neuritis. Arch Neurol 69:752–756. https://doi.org/10.1001/archneurol.2011.2956
Oliveira LM, Apóstolos-Pereira SL, Pitombeira MS, Bruel Torretta PH, Callegaro D, Sato DK (2018) Persistent MOG-IgG positivity is a predictor of recurrence in MOG-IgG-associated optic neuritis, encephalitis and myelitis. Mult Scler J 25:1907–1914. https://doi.org/10.1177/1352458518811597
Jurynczyk M, Messina S, Woodhall MR, Raza N, Everett R, Roca-Fernandez A, Tackley G, Hamid S, Sheard A, Reynolds G, Chandratre S, Hemingway C, Jacob A, Vincent A, Leite MI, Waters P, Palace J (2017) Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain. 140:3128–3138. https://doi.org/10.1093/brain/awx276
Cobo-Calvo A, Ruiz A, Maillart E, Audoin B, Zephir H, Bourre B, Ciron J, Collongues N, Brassat D, Cotton F, Papeix C, Durand-Dubief F, Laplaud D, Deschamps R, Cohen M, Biotti D, Ayrignac X, Tilikete C, Thouvenot E, Brochet B, Dulau C, Moreau T, Tourbah A, Lebranchu P, Michel L, Lebrun-Frenay C, Montcuquet A, Mathey G, Debouverie M, Pelletier J, Labauge P, Derache N, Coustans M, Rollot F, de Seze J, Vukusic S, Marignier R, OFSEP and NOMADMUS Study Group (2018) Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults. Neurology. 90:e1858–e1869. https://doi.org/10.1212/wnl.0000000000005560
Pröbstel AK, Dornmair K, Bittner R, Sperl P, Jenne D, Magalhaes S et al (2011) Antibodies to MOG are transient in childhood acute disseminated encephalomyelitis. Neurology. 77:580–588. https://doi.org/10.1212/WNL.0b013e318228c0b1
Duignan S, Wright S, Rossor T, Cazabon J, Gilmour K, Ciccarelli O, Wassmer E, Lim M, Hemingway C, Hacohen Y (2018) Myelin oligodendrocyte glycoprotein and aquaporin-4 antibodies are highly specific in children with acquired demyelinating syndromes. Dev Med Child Neurol 60:958–962. https://doi.org/10.1111/dmcn.13703
Haase CG, Guggenmos J, Brehm U, Andersson M, Olsson T, Reindl M, Schneidewind JM, Zettl UK, Heidenreich F, Berger T, Wekerle H, Hohlfeld R, Linington C (2001) The fine specificity of the myelin oligodendrocyte glycoprotein autoantibody response in patients with multiple sclerosis and normal healthy controls. J Neuroimmunol 114:220–225. https://doi.org/10.1016/S0165-5728(00)00462-8
Pittock SJ, Reindl M, Achenbach S, Berger T, Bruck W, Konig F, Morales Y, Lassmann H, Bryant S, Moore SB, Keegan BM, Lucchinetti CF (2007) Myelin oligodendrocyte glycoprotein antibodies in pathologically proven multiple sclerosis: frequency, stability and clinicopathologic correlations. Mult Scler 13:7–16. https://doi.org/10.1177/1352458506072189
Pelayo R, Tintoré M, Montalban X, Rovira A, Espejo C, Reindl M, Berger T (2007) Antimyelin antibodies with no progression to multiple sclerosis. N Engl J Med 356:426–428. https://doi.org/10.1056/NEJMc062467
Waters PJ, Komorowski L, Woodhall M, Lederer S, Majed M, Fryer J, Mills J, Flanagan EP, Irani SR, Kunchok AC, McKeon A, Pittock SJ (2019) A multicenter comparison of MOG-IgG cell-based assays. Neurology. 92:e1250–e1255. https://doi.org/10.1212/WNL.0000000000007096
Peng Y, Liu L, Zheng Y, Qiao Z, Feng K, Wang J (2018) Diagnostic implications of MOG/AQP4 antibodies in recurrent optic neuritis. Exp Ther Med 16:950–958. https://doi.org/10.3892/etm.2018.6273
Jacobsen LB, Calvin SA, Lobenhofer EK (2009) Transcriptional effects of transfection: the potential for misinterpretation of gene expression data generated from transiently transfected cells. Biotechniques. 47:617–624. https://doi.org/10.2144/000113132
Hennes E-M, Baumann M, Lechner C, Rostásy K (2018) MOG Spectrum disorders and role of MOG-antibodies in clinical practice. Neuropediatrics. 49:3–11. https://doi.org/10.1055/s-0037-1604404
Waters P, Woodhall M, O’Connor KC, Reindl M, Lang B, Sato DK et al (2015) MOG cell-based assay detects non-MS patients with inflammatory neurologic disease. Neurol Neuroimmunol NeuroInflammation. 2:1–11. https://doi.org/10.1212/NXI.0000000000000089
Gerdes H-H, Kaether C (1996) Green fluorescent protein: applications in cell biology. FEBS Lett 389:44–47. https://doi.org/10.1016/0014-5793(96)00586-8
Pines J (1995) GFP in mammalian cells. Trends Genet 11:326–327. https://doi.org/10.1016/S0168-9525(00)89092-7
Ohba Y, Fujioka Y, Nakada S, Tsuda M (2013) Fluorescent protein-based biosensors and their clinical applications. Prog Mol Biol Transl Sci 113:313–348. https://doi.org/10.1016/B978-0-12-386932-6.00008-9
Liu H-S, Jan M-S, Chou C-K, Chen P-H, Ke N-J (1999) Is green fluorescent protein toxic to the living cells? Biochem Biophys Res Commun 260:712–717. https://doi.org/10.1006/BBRC.1999.0954
Mader S, Lutterotti A, Di Pauli F, Kuenz B, Schanda K, Aboul-Enein F et al (2010) Patterns of antibody binding to aquaporin-4 isoforms in neuromyelitis optica. PLoS One 5:1–7. https://doi.org/10.1371/journal.pone.0010455
Dash AK, Yende AS, Tyagi RK (2017) Novel application of red fluorescent protein (DsRed-express) for the study of functional dynamics of nuclear receptors. J Fluoresc 27:1225–1231. https://doi.org/10.1007/s10895-017-2109-z
Waters PJ, Pittock SJ, Bennett JL, Jarius S, Weinshenker BG, Wingerchuk DM (2014) Evaluation of aquaporin-4 antibody assays. Clin Exp Neuroimmunol 5:290–303. https://doi.org/10.1111/cen3.12107
Cobo-Calvo A, Sepúlveda M, Rollot F, Armangué T, Ruiz A, Maillart E, Papeix C, Audoin B, Zephir H, Biotti D, Ciron J, Durand-Dubief F, Collongues N, Ayrignac X, Labauge P, Thouvenot E, Bourre B, Montcuquet A, Cohen M, Deschamps R, Solà-Valls N, Llufriu S, de Seze J, Blanco Y, Vukusic S, Saiz A, Marignier R (2019) Evaluation of treatment response in adults with relapsing MOG-Ab-associated disease. J Neuroinflammation 16:1–12. https://doi.org/10.1186/s12974-019-1525-1
Pedreño M, Sepúlveda M, Armangué T, Sabater L, Martínez-Hernandez E, Arrambide G, Blanco Y, Llufriu S, Martínez-Lapiscina EH, Mulero P, Sola-Valls N, Ruiz-García R, Tintoré M, Dalmau J, Graus F, Saiz A (2019) Frequency and relevance of IgM, and IgA antibodies against MOG in MOG-IgG-associated disease. Mult Scler Relat Disord. 28:230–234. https://doi.org/10.1016/j.msard.2019.01.007
Mariotto S, Ferrari S, Monaco S, Benedetti MD, Schanda K, Alberti D, Farinazzo A, Capra R, Mancinelli C, de Rossi N, Bombardi R, Zuliani L, Zoccarato M, Tanel R, Bonora A, Turatti M, Calabrese M, Polo A, Pavone A, Grazian L, Sechi GP, Sechi E, Urso D, Delogu R, Janes F, Deotto L, Cadaldini M, Bianchi MR, Cantalupo G, Reindl M, Gajofatto A (2017) Clinical spectrum and IgG subclass analysis of anti-myelin oligodendrocyte glycoprotein antibody-associated syndromes: a multicenter study. J Neurol 264:2420–2430. https://doi.org/10.1007/s00415-017-8635-4
Gastaldi M, Scaranzin S, Jarius S, Wildeman B, Zardini E, Mallucci G, Rigoni E, Vegezzi E, Foiadelli T, Savasta S, Banfi P, Versino M, Benedetti L, Novi G, Mancardi MM, Giacomini T, Annovazzi P, Baroncini D, Ferraro D, Lampasona V, Reindl M, Waters P, Franciotta D (2020) Cell-based assays for the detection of MOG antibodies: a comparative study. J Neurol:1–10. https://doi.org/10.1007/s00415-020-10024-0
Kim Y, Hyun J-W, Woodhall MR, Oh Y-M, Lee J-E, Jung JY, Kim SY, Lee MY, Kim SH, Kim W, Irani SR, Waters P, Choi K, Kim HJ (2020) Refining cell-based assay to detect MOG-IgG in patients with central nervous system inflammatory diseases. Mult Scler Relat Disord. 40:1–9. https://doi.org/10.1016/j.msard.2020.101939
Sugimoto K, Mori M, Liu J, Tanaka S, Kaneko K, Oji S, Takahashi T, Uzawa A, Uchida T, Masuda H, Ohtani R, Nomura K, Hiwasa T, Kuwabara S (2019) The accuracy of flow cytometric cell-based assay to detect anti-myelin oligodendrocyte glycoprotein (MOG) antibodies determining the optimal method for positivity judgement. J Neuroimmunol 336:1–9. https://doi.org/10.1016/j.jneuroim.2019.577021
Waters P, Reindl M, Saiz A, Schanda K, Tuller F, Kral V, Nytrova P, Sobek O, Nielsen HH, Barington T, Lillevang ST, Illes Z, Rentzsch K, Berthele A, Berki T, Granieri L, Bertolotto A, Giometto B, Zuliani L, Hamann D, van Pelt ED, Hintzen R, Höftberger R, Costa C, Comabella M, Montalban X, Tintoré M, Siva A, Altintas A, Deniz G, Woodhall M, Palace J, Paul F, Hartung HP, Aktas O, Jarius S, Wildemann B, Vedeler C, Ruiz A, Leite MI, Trillenberg P, Probst M, Saschenbrecker S, Vincent A, Marignier R (2016) Multicentre comparison of a diagnostic assay: Aquaporin-4 antibodies in neuromyelitis optica. J Neurol Neurosurg Psychiatry 87:1005–1015. https://doi.org/10.1136/jnnp-2015-312601
Reindl M, Schanda K, Woodhall M, Tea F, Ramanathan S, Sagen J, Fryer JP, Mills J, Teegen B, Mindorf S, Ritter N, Krummrei U, Stöcker W, Eggert J, Flanagan EP, Ramberger M, Hegen H, Rostasy K, Berger T, Leite MI, Palace J, Irani SR, Dale RC, Probst C, Probst M, Brilot F, Pittock SJ, Waters P (2020) An international multicenter examination of MOG antibody assays. Neuroimmunol Neuroinfllammation 7:1–12. https://doi.org/10.1212/nxi.0000000000000674
Funding
This review is based on research supported by the Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior, Brasil (CAPES), Finance Code 001.
Author information
Authors and Affiliations
Contributions
All authors contributed to the manuscript preparation and wrote, read, and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AM has received a scholarship from CAPES/Brazil.
MW has no disclosures.
PW and the University of Oxford hold patents and receive royalties for antibody assays. PW has received honoraria from Alexion and UBC and a research grant from Euroimmun AG.
DKS has received a scholarship from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan; a Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (KAKENHI 15K19472); research support from CNPq/Brazil (425331/2016-4), FAPERGS/MS/CNPq/SESRS (17/2551-0001391-3) PPSUS/Brazil, TEVA (research grant for EMOCEMP Investigator Initiated Study), and Euroimmun AG (Neuroimmunological Complications associated with Arboviruses); and speaker honoraria from Biogen, Novartis, Genzyme, TEVA, Merck Serono, Roche, and Bayer and has participated in advisory boards for Shire, Roche, TEVA, Merck Serono, and Quest/Athena Diagnostics.
Ethics approval
Not applicable.
Informed consent
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marchionatti, A., Woodhall, M., Waters, P.J. et al. Detection of MOG-IgG by cell-based assay: moving from discovery to clinical practice. Neurol Sci 42, 73–80 (2021). https://doi.org/10.1007/s10072-020-04828-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-020-04828-1