Abstract
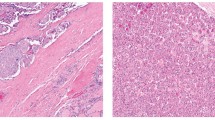
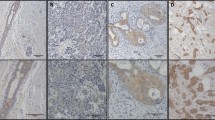
Epigenetic dysregulation is a hallmark of cancer, and aberrant methylation of cytosine residues plays a crucial role in abnormal gene expression in cancer cells. Recent studies demonstrate that 5-hydroxymethylcytosine (5-hmC) generated through 5-methylcytosine (5-mC) oxidation is significantly depleted in various cancers. However, whether 5-hmC levels change during the stepwise progression of thyroid carcinoma and the mechanisms underlying this effect remain unknown. The aims of this study were (i) to assess 5-hmC levels in normal and cancerous thyroid tissues, and (ii) identify clinicopathologic and genetic factors associated with the dysregulated hydroxymethylation of cytosine. Enzyme-linked immunosorbent assay (ELISA) showed that 5-hmC was significantly reduced in TERT promoter-mutated papillary thyroid carcinomas (PTCs) and anaplastic thyroid carcinomas (ATCs), while there was no significant difference in 5-hmC levels between TERT promoter-wild-type PTCs and normal thyroid tissues. Results of semi-quantitative analysis of 5-hmC through immunohistochemistry correlated well with those of ELISA and confirmed the loss of 5-hmC in tumor cells. Immunohistochemistry confirmed lower 5-hmC positivity in TERT promoter-mutated PTCs (n = 10) and ATCs (n = 4) than in normal thyroid tissues (n = 8) and TERT promoter-wild-type PTCs (n = 63). Tumor size (> 1 cm) and advanced stage were associated with decreased global 5-hmC in PTCs, while age, gross extrathyroidal invasion, node metastasis, and BRAF mutation were not. Collectively, these findings demonstrated that loss of 5-hmC is an epigenetic hallmark of thyroid carcinomas with TERT promoter mutation, indicating that TERT promoter-mutated thyroid carcinoma has a distinct molecular profile.





Similar content being viewed by others
References
Cabanillas ME, McFadden DG, Durante C (2016) Thyroid cancer. Lancet 388:2783–2795. https://doi.org/10.1016/S0140-6736(16)30172-6
Huang FW, Hodis E, Xu MJ, et al (2013) Highly recurrent TERT promoter mutations in human melanoma. Science 339:957–9. https://doi.org/10.1126/science.1229259
Horn S, Figl A, Rachakonda PS, et al (2013) TERT promoter mutations in familial and sporadic melanoma. Science 339:959–61. https://doi.org/10.1126/science.1230062
Bullock M, Lim G, Zhu Y, et al (2019) ETS Factor ETV5 Activates the Mutant Telomerase Reverse Transcriptase Promoter in Thyroid Cancer. Thyroid 29:1623–1633. https://doi.org/10.1089/thy.2018.0314
Akıncılar SC, Khattar E, Boon PLS, et al (2016) Long-Range Chromatin Interactions Drive Mutant TERT Promoter Activation. Cancer Discov 6:1276–1291. https://doi.org/10.1158/2159-8290.CD-16-0177
Melo M, da Rocha AG, Vinagre J, et al (2014) TERT Promoter Mutations Are a Major Indicator of Poor Outcome in Differentiated Thyroid Carcinomas. J Clin Endocrinol Metab 99:E754–E765. https://doi.org/10.1210/jc.2013-3734
Vuong HG, Altibi AM, Duong UN, et al (2017) Role of molecular markers to predict distant metastasis in papillary thyroid carcinoma: Promising value of TERT promoter mutations and insignificant role of BRAF mutations—a meta-analysis. Tumor Biol 39:101042831771391. https://doi.org/10.1177/1010428317713913
Oishi N, Kondo T, Nakazawa T, et al (2017) Frequent BRAF V600E and Absence of TERT Promoter Mutations Characterize Sporadic Pediatric Papillary Thyroid Carcinomas in Japan. Endocr Pathol 28:103–111. https://doi.org/10.1007/s12022-017-9470-y
Liu R, Xing M (2016) TERT promoter mutations in thyroid cancer. Endocr Relat Cancer 23:R143-55. https://doi.org/10.1530/ERC-15-0533
Landa I, Ganly I, Chan T, et al (2013) Frequent Somatic TERT Promoter Mutations in Thyroid Cancer: Higher Prevalence in Advanced Forms of the Disease. J Clin Endocrinol Metab 98:E1562–E1566. https://doi.org/10.1210/jc.2013-2383
Oishi N, Kondo T, Ebina A, et al (2017) Molecular alterations of coexisting thyroid papillary carcinoma and anaplastic carcinoma: identification of TERT mutation as an independent risk factor for transformation. Mod Pathol 30:1527–1537. https://doi.org/10.1038/modpathol.2017.75
Cancer Genome Atlas Research Network (2014) Integrated genomic characterization of papillary thyroid carcinoma. Cell 159:676–90. https://doi.org/10.1016/j.cell.2014.09.050
Kondo T, Nakazawa T, Ma D, et al (2009) Epigenetic silencing of TTF-1/NKX2-1 through DNA hypermethylation and histone H3 modulation in thyroid carcinomas. Lab Investig 89:791–799. https://doi.org/10.1038/labinvest.2009.50
Lian CG, Xu Y, Ceol C, et al (2012) Loss of 5-hydroxymethylcytosine is an epigenetic hallmark of melanoma. Cell 150:1135–46. https://doi.org/10.1016/j.cell.2012.07.033
Pronier E, Almire C, Mokrani H, et al (2011) Inhibition of TET2-mediated conversion of 5-methylcytosine to 5-hydroxymethylcytosine disturbs erythroid and granulomonocytic differentiation of human hematopoietic progenitors. Blood 118:2551–5. https://doi.org/10.1182/blood-2010-12-324707
Ko M, Huang Y, Jankowska AM, et al (2010) Impaired hydroxylation of 5-methylcytosine in myeloid cancers with mutant TET2. Nature 468:839–43. https://doi.org/10.1038/nature09586
Kraus TFJ, Globisch D, Wagner M, et al (2012) Low values of 5-hydroxymethylcytosine (5hmC), the “sixth base,” are associated with anaplasia in human brain tumors. Int J cancer 131:1577–90. https://doi.org/10.1002/ijc.27429
Tong M, Gao S, Qi W, et al (2019) 5‑Hydroxymethylcytosine as a potential epigenetic biomarker in papillary thyroid carcinoma. Oncol Lett 18:2304–2309. https://doi.org/10.3892/ol.2019.10531
Liu R, Bishop J, Zhu G, et al (2017) Mortality Risk Stratification by Combining BRAF V600E and TERT Promoter Mutations in Papillary Thyroid Cancer. JAMA Oncol 3:202. https://doi.org/10.1001/jamaoncol.2016.3288
Paulsson JO, Olander A, Haglund F, et al (2018) TERT Immunohistochemistry Is a Poor Predictor of TERT Promoter Mutations and Gene Expression in Follicular Thyroid Carcinoma. Endocr Pathol 29:380–383. https://doi.org/10.1007/s12022-018-9551-6
Zhu X, Dresser K, Chen BJ (2019) Loss of 5‐hydroxymethylcytosine immunohistochemical expression is a useful diagnostic aid for distinguishing hepatocellular carcinoma in cytology fine needle aspiration specimens. Cytopathology 30:492–498. https://doi.org/10.1111/cyt.12719
Haglund F, Juhlin CC, Brown T, et al (2015) TERT promoter mutations are rare in parathyroid tumors. Endocr Relat Cancer 22:L9–L11. https://doi.org/10.1530/ERC-15-0121
Barazeghi E, Gill AJ, Sidhu S, et al (2016) 5-Hydroxymethylcytosine discriminates between parathyroid adenoma and carcinoma. Clin Epigenetics 8:31. https://doi.org/10.1186/s13148-016-0197-2
Kroeze LI, van der Reijden BA, Jansen JH (2015) 5-Hydroxymethylcytosine: An epigenetic mark frequently deregulated in cancer. Biochim Biophys Acta - Rev Cancer 1855:144–154. https://doi.org/10.1016/j.bbcan.2015.01.001
Pfeifer GP, Kadam S, Jin S-G (2013) 5-hydroxymethylcytosine and its potential roles in development and cancer. Epigenetics Chromatin 6:10. https://doi.org/10.1186/1756-8935-6-10
Lemonnier F, Couronne L, Parrens M, et al (2012) Recurrent TET2 mutations in peripheral T-cell lymphomas correlate with TFH -like features and adverse clinical parameters. Blood 120:1466–1470. https://doi.org/10.1182/blood-2012-02-408542
Lemonnier F, Poullot E, Dupuy A, et al (2018) Loss of 5-hydroxymethylcytosine is a frequent event in peripheral T-cell lymphomas. Haematologica 103:e115–e118. https://doi.org/10.3324/haematol.2017.167973
Figueroa ME, Abdel-Wahab O, Lu C, et al (2010) Leukemic IDH1 and IDH2 Mutations Result in a Hypermethylation Phenotype, Disrupt TET2 Function, and Impair Hematopoietic Differentiation. Cancer Cell 18:553–567. https://doi.org/10.1016/j.ccr.2010.11.015
Dang L, White DW, Gross S, et al (2009) Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462:739–744. https://doi.org/10.1038/nature08617
Couronné L, Bastard C, Bernard OA (2012) TET2 and DNMT3A Mutations in Human T-Cell Lymphoma. N Engl J Med 366:95–96. https://doi.org/10.1056/NEJMc1111708
Murugan AK, Bojdani E, Xing M (2010) Identification and functional characterization of isocitrate dehydrogenase 1 (IDH1) mutations in thyroid cancer. Biochem Biophys Res Commun 393:555–559. https://doi.org/10.1016/j.bbrc.2010.02.095
Hemerly JP, Bastos AU, Cerutti JM (2010) Identification of several novel non-p.R132 IDH1 variants in thyroid carcinomas. Eur J Endocrinol 163:747–755. https://doi.org/10.1530/EJE-10-0473
Rakheja D, Boriack RL, Mitui M, et al (2011) Papillary thyroid carcinoma shows elevated levels of 2-hydroxyglutarate. Tumor Biol 32:325–333. https://doi.org/10.1007/s13277-010-0125-6
Shenoy N, Creagan E, Witzig T, Levine M (2018) Ascorbic Acid in Cancer Treatment: Let the Phoenix Fly. Cancer Cell 1–7. https://doi.org/10.1016/j.ccell.2018.07.014
Shenoy N, Bhagat TD, Cheville J, et al (2019) Ascorbic acid-induced TET activation mitigates adverse hydroxymethylcytosine loss in renal cell carcinoma. J Clin Invest 130:1612–1625. https://doi.org/10.1172/JCI98747
Acknowledgments
We thank Ms. Wakaba Iha, Mr. Yoshihito Koshimizu, and Mr. Tadashi Iwato for technical support, and Ms. Kayoko Kono for executive assistance.
Funding
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 19K07412.
This study was approved by the Institutional Review Board of the University of Yamanashi.
Author information
Authors and Affiliations
Contributions
NO designed the work, performed the analyses, interpreted data, and drafted the manuscript. HGV performed analysis and interpreted data. KM and TK interpreted data and supervised the research. All the authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics approval
Approved by the Institutional Review Board of the University of Yamanashi.
Consent to participate
Approved with the use of an opt-out methodology based on the low risk to the patients.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oishi, N., Vuong, H.G., Mochizuki, K. et al. Loss of 5-Hydroxymethylcytosine is an Epigenetic Hallmark of Thyroid Carcinomas with TERT Promoter Mutations. Endocr Pathol 31, 359–366 (2020). https://doi.org/10.1007/s12022-020-09652-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-020-09652-z