Abstract
Beta-cyclodextrin was used as structure directing agent for the formation of a novel small, uniform platinum nanoparticles. The resultant platinum nanoparticles were supported over multi-walled carbon nanotubes (PtMWCNTs) and used as catalyst for hydrogen generation. The resultant composite was characterized via X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), transmission electron microscopy (TEM), scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS). The TEM displayed the diameter of MWCNT ranged from 20 to 35 nm, and the average size of Pt nanoparticles was 2.8 nm. Testing of catalytic activity showed that the highest rate achieved was 21.2 ml min−1gcat−1 at 303 K with pH 7 and 835 μmoles of NaBH4. In the pH adjusted trials, the greatest rate achieved was 16.9 ml min−1gcat−1 outperforming both pH 7 and pH 6 despite the known increase in hydrolysis rate at lower pH. The activation energy of the reaction as catalyzed by the PtMWCNTs was calculated to be 46.2 kJ mol−1, which is competitive when compared to other heterogeneous catalysts reported to date. The lower activation energy results from the small Pt nanoparticles (∼3 nm) that create more active sites to adsorb the borohydride ion and facilitate the generation of hydride ions via electron transfer. The generated hydride ions immediately combine with the protons generated from the weakened H−O−H bond to produce hydrogen gas. The MWCNTs composite displayed its durability and stability though its reusable test that generated 15.2 ml of hydrogen.
Export citation and abstract BibTeX RIS
Many different fuels are being researched, tested, and applied to overcome the world's dependence on coal and oil.1,2 These two energy sources, while having been widely used for over a century, are non-renewable and are leading contributors to anthropogenic climate change. Hydrogen gas is another alternative fuel that has similar characteristics to natural gas, such as high-energy density and already designed infrastructure for its application, which contributes to it becoming an increasingly viable alternative fuel.3–7 Hydrogen gas combustion releases water, making it a safer fuel for wide-spread use, and many transportation companies have developed hydrogen fuel cell vehicles. While there are many methods being researched and optimized to produce hydrogen gas, there is the problem of storage across the methods. Taking advantage of the high-energy density of hydrogen gas requires pressurization of the fuel, which not only increases the energy intensity of fuel production but also increases the hazards of its application.
One method to overcome the storage problems of hydrogen gas is the use of hydrogen feedstock materials (HFMs). HFMs are compounds that controllably release hydrogen gas but store it at ambient conditions at higher densities. Metal hydrides are one family of these compounds that are commonly used as HFMs. One such metal hydride, sodium borohydride, has one of the highest hydrogen densities at 10.8% wt, is environmentally benign, and can store or release hydrogen gas under ambient conditions.8 Sodium borohydride hydrolyzes under aqueous conditions to release hydrogen gas, but this reaction proceeds too slowly to be relevant for energy applications. Thus, there has been much research into discovering a proper catalyst for this reaction.8–12 Many promising catalysts are at least partially composed of transition metals, and some of the best performing of the catalysts are of the platinum group.13,14
The use of platinum as the catalyst for the hydrolysis of sodium borohydride shows great promise.15 Platinum is used in many hydrogen production methods due to its chemical stability and conductivity, which is beneficial in the multi-electron processes that are often involved in hydrogen evolution.16 However, platinum is expensive, which limits scaling most catalysts to an industrial level. The use of platinum nanoparticles may help to overcome the cost of scaling as the better atom economy of nanoparticles provides a greater surface area to volume ratio, reducing the overall amount of platinum needed in a catalyst. Unfortunately, the platinum nanoparticles would be difficult to reuse and reclaim alone. Thus, the use of a support structure to dock the nanoparticles unto is proposed.
Multi-walled carbon nanotubes (MWCNTs) including high surface area carbon materials have shown promise as support structures due to their ability to be functionalized by many chemical species and their high-strength and conductivity.17–20 Further, MWCNTs have demonstrated high chemical stability, which is important due to the oxidative and reductive conditions produced by NaBH4 hydrolysis.20–23 Finally, MWCNTs including carbon nanofibers maintain the high-surface area required for a successful catalyst, and their hydrophobic nature allows for the material to be reclaimed more easily than other nanomaterials.24–26
In his study, we use a greenultrafine platinum nanoparticles functionalized MWCNTs to enhance the hydrogen production. The catalytic ability and reusability of platinum nanoparticles supported over MWCNTs (PtMWCNTs) were investigated. The efficiency of nanocomposite was evaluated in various temperature pH, and concentration conditions to demonstrate its catalytic ability in improving the hydrogen production.
Experimental
All materials were purchased from Sigma Aldrich with high purity unless otherwise specified.
Synthesis
Chloroplatinic acid was used to create a 1 mM precursor platinum solution. This solution was then added to a separate 10 mM aqueous beta-cyclodextrin solution in a 5:28 volume ratio and was stirred for ten minutes. A fresh solution of 180 mM NaBH4 was then prepared and added to the mixture and the resulting 134.5 μM metal solution is stirred for 2 h to facilitate nanoparticle formation. To produce the composites, 1 ml of the resulting nanoparticle solution is added to 50 mg of multi-walled carbon nanotubes (MWCNTs) to allow functionalization via incipient wetness impregnation.20–23 The produced mixture was then heated to 333 K and maintained this temperature for 48 h to promote the evaporation of excess water. The MWCNTs used in this study were produced by CVD of methane over iron-based catalysts.20–22
Characterization
The reported diffraction peaks of MWCNTs and PtMWCNTs were confirmed through powder X-ray diffraction (P-XRD Rigaku Miniflex II). The samples were scanned at the angles from 5° to 90°.
The presence of beta-cyclodextrin and MWCNTs was identified by using Fourier transform infrared spectroscopy (FTIR, Shimadzu IR-Tracer 100) with an Attenuated Total Reflectance (ATR, Shimadzu QATR-S) attachment.
Transmission electron microscopy (TEM JEM-2100F)) was used to determine the dimensions of the nanoparticles produced in the study. Samples were prepared by pipetting 1 μl of the composite suspended in methanol onto a TEM sample grid and dried in an oven overnight.
Scanning electron microscopy (SEM) was used to determine the morphology at a microscale. Energy dispersive spectroscopy (EDS) was used to investigate the elements present and to quantify the loading of the platinum into the composite. The composite material was loaded on a sample holder using carbon-tape and analyzed under FE-SEM (Hitachi 4700) with EDAX attachment.
Catalysis
Catalytic activity of the produced PtMWCNTs composite was tested using a gravimetric water displacement system.7,20–23,27 Each reaction was run in 100 ml of deionized water with 10 mg of catalyst at 295 K, pH 7, and with 835 μmoles of NaBH4 (J.T. Baker) unless otherwise noted. Variations were made to the pH using HCl and NaOH to adjust pH to 6 and 8 respectively, and to the temperature of the trial using an ice bath for the 273 K trial and a hot plate for the 303 K trial. Concentration of reactant was also varied, lowered to 635 μmoles and raised to 1035 μmoles. Stirring via a magnetic stir bar was used throughout the trials to maintain suspension of the composites with the exception to the temperature altered trials, which required insulation to maintain the temperature throughout the trials. The water displaced by the hydrogen evolved was gravimetrically quantified using an Ohaus Pioneer Balance (Pa124) with mass logging software. Rate was determined as a product of the overall hydrogen gas produced, the inverse of the duration of the trials (2 h), and the mass or volume for the catalyst used. The reusability of the composites was examined by the same gravimetric system, PtMWCNTs was used to catalyzed the reaction for 10 h, and 835 μmoles of NaBH4 was added for every 2 h.
Results and Discussion
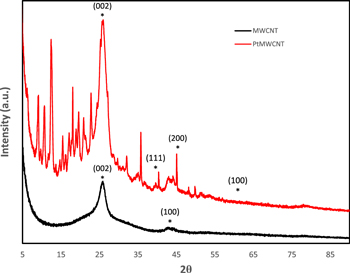
Figure 1 illustrated the P-XRD pattern of MWCNTs and PtMWCNTs. The diffraction angles of MWCNTs was observed at 26° and 42° which are corresponded to the (002) and (100) planes.28,29 Multiple peaks patterns from 7° to 22° indicated the presence of beta-cyclodextrin in PtMWCNTs.30 The P-XRD peaks at 39°, 46° and 67° reflected the (111), (200), and (220) plane structure of platinum nanoparticles.31,32
Figure 1. P-XRD pattern of MWCNTs and PtMWCNTs.
Download figure:
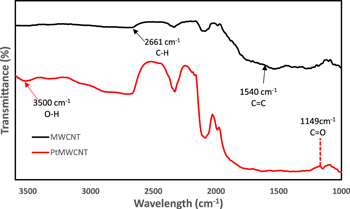
Standard image High-resolution imageFigure 2 showed the FTIR spectrum of MWCNTs and PtMWCNTs. The peaks at 1056 cm−1, 1540 cm−1, 1742 cm−1, and 2661 cm−1 displayed the functional group of MWCNTs.28,29 The other peaks at 1742 cm−1 and 2661 cm−1 were ascribed to the C=O group and C–H stretching.33,34 In the FTIR of PtMWCNTs, the presence of beta-cyclodextrin was identified through the peaks at 3500 cm−1 and 1149 cm−1 which were corresponded to the OH vibration and the C=O group of glycosidic bond.35,36
Figure 2. FTIR of MWCNTs and PtMWCNTs.
Download figure:
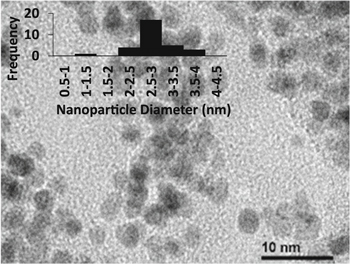
Standard image High-resolution imageTEM micrographs in Fig. 3 confirmed the presence of uniform platinum nanoparticles (PtNPs) within the sample. Nanoparticles were observed with diameters of 2.8 ± 0.58 nm.
Figure 3. TEM micrographs of the platinum nanoparticles (PtNPs) depicting an average diameter of 2.8 ± 0.58 nm.
Download figure:
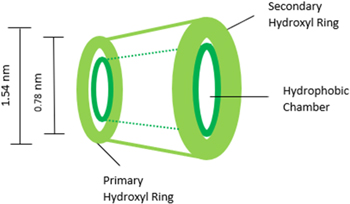
Standard image High-resolution imageBeta-cyclodextrin act as the capping agent and directing structure agent in the synthesis of the platinum nanoparticles to control the diameter of Pt nanoparticles in the range of 3 nm. Because β-cyclodextrin has unique confirmation (Fig. 4) consisting of layered internal cavities, beta-cyclodextrin aids in restricting the final shape of particles and leading to isolated shapes.12 The conical-like structure of the interior apolar cavity aids in confining the nanoparticle throughout its formation leading to isolated, precise shapes and diameter.12
Figure 4. Graphical depiction of the capping agent β-cyclodextrin illustrating primary and secondary hydroxyl rings.
Download figure:
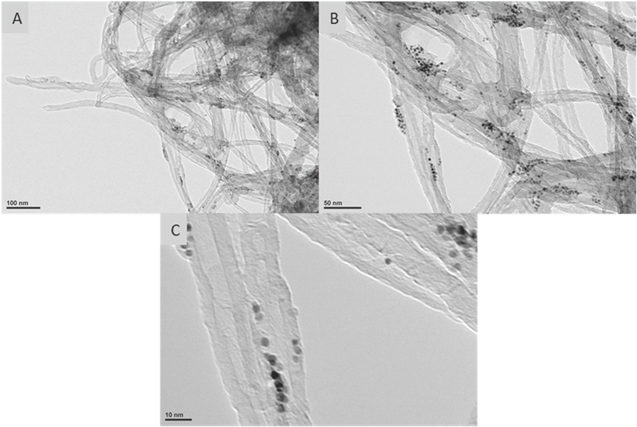
Standard image High-resolution imageTEM micrographs in Fig. 5 show the synthesized PtMWCNTs composite at three magnifications. Beta-cyclodextrin was selected and used as a capping agent to combat the tendency of nanoparticles to agglomerate. In a study by Berhault and Kochkar, it was found that the unique shape of beta-cyclodextrin aids in restricting the final morphologies of silver and platinum nanoparticles.12 The interior cavities of the agent act to promote the hydrophobic-hydrophobic interactions of nanoparticles. These interactions lead to the formation of isolated particles formed of aggregated primary nanoparticles.12 Figure 5A shows the relative coverage of the Pt nanoparticles, occurring throughout the MWCNTs. Figure 5B resolves that these clusters of platinum are individual nanoparticles supported over the surface of MWCNTs. Figure 3C allows the measurement of the components of the materials, depicting the 20 to 35 nm diameter of the MWCNTs and highly dispersed ultrafine (∼3 nm diameter) platinum nanoparticles.
Figure 5. TEM micrographs of the PtMWCNTs composite. 5A and 5B depicts the functionalization of the nanoparticles within the composite. 3C displays the size of the original component materials used in the synthesis. The micrographs 5A–5C have scale bars of 100 nm, 50 nm, and 10 nm, respectively.
Download figure:
Standard image High-resolution imageFigure 6. SEM micrograph and the corresponding EDS table of the imaged area.
Download figure:
Standard image High-resolution imageSEM micrograph in Fig. 6 shows the synthesized PtMWCNTs composite at 50 k magnification and the resulting EDS spectrum of the displayed area and the loading of platinum into the sample is 0.0525% wt.
The composite increased the production of hydrogen from the hydrolysis of sodium borohydride by almost 75% when compared to the precursor platinum nanoparticles (Fig. 7). The composite (PtMWCNTs) evolving hydrogen at a rate of 14.6 ml·min−1·gcat−1 and only 0.43 ml·min−1·mlcat−1 for the PtNPs. This may be explained by the increased density of the composite due to the presence of platinum nanoparticles, which would lessen catalyst used (per mass) compared to pure MWCNTs.
Figure 7. Comparison of the volume of hydrogen gas vs time generated by the hydrolysis reaction of sodium borohydride as catalyzed by the produced PtNPs and PtMWCNTs. Each reaction was run at 295 K, pH 7, and with 835 μmoles of NaBH4.
Download figure:
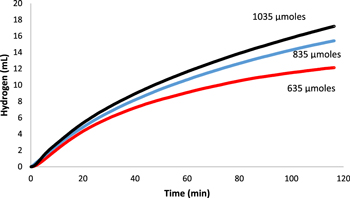
Standard image High-resolution imageIn the reactant concentration varied trials, the 1035 μmoles condition produced hydrogen at the greatest rate of 17.2 ml·min−1·gcat−1. In the lower concentrations' hydrogen gas was produced at 15.4 ml·min−1·gcat−1 and 12.1 ml·min−1·gcat−1 for the 835 and 635 μmole conditions respectively (Fig. 8). The data showed the trend of increasing rate of hydrogen generation as concentration of reactant increased. This implies the optimal concentration for 10 mg of catalyst is higher than 1035 μmoles in 100 ml of solution, as previous studies with PtMWCNTs have shown a decrease in hydrogen generation rates with increased reactant concentration. The observed trend of an optimal concentration where reaction rate is negatively affected by altering reactant concentration may be the cause of the diminishing increase of hydrogen evolution rate seen in Fig. 8 as concentration increases. This is theorized to be caused by increased solution viscosity due to increased NaBH4 concentrations.21,22
Figure 8. Comparison of the volume of hydrogen gas vs time generated by the hydrolysis reaction of sodium borohydride as catalyzed by the produced PtMWCNTs composite under varied concentrations of NaBH4 (635 μmoles, 835 μmoles, 1035 μmoles).
Download figure:
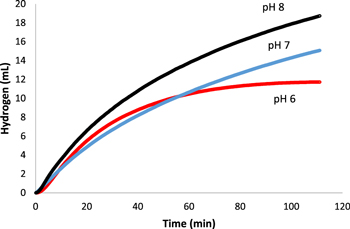
Standard image High-resolution imageWhen catalytic activity was tested under altered pH, pH 8 unexpectedly generated hydrogen at the greatest rate of 16.9 ml·min−1·gcat−1 outperforming pH 7 and pH 6 with rates of 13.6 ml·min−1·gcat−1 and 10.6 ml·min−1·gcat−1 respectively (Fig. 9). This is unexpected because of the known increase to the rate of hydrolysis at lower pHs.10,37 The production of BO2− ions in the reaction causes the pH sensitivity of the reaction, as OH– ion shift the equilibrium of the reaction to the reactants, and H+ shifting to the products as per Eq. 1.
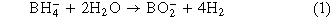
Therefore, the catalyst is suspected to be more affected by the pH shift than the reactants. This result is supported by previous studies that have shown similarly produced AuMWCNTs and AgMWCNTs composites are pH sensitive, performing better at neutral pH, showing a similar competition between best working pH for the reactants vs the catalyst.21,22 The platinum catalyst showed increased reactivity under basic conditions. Similar findings have been reported in the hydrolysis of sodium borohydride.38,39 In the presence of OH– ions at pH 8, the reconstruction of HO–BH3– anion is invoked as an alternative to BH3– dissociation, with the assumption that BH4– and (HO)n–BH(4−n)− are equally reactive at the catalytic site.38
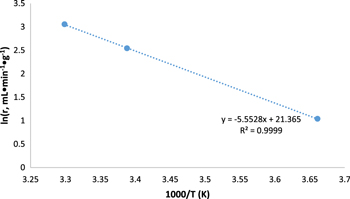
Increasing temperature resulted in increasing rates of hydrogen production with 303 K displaying a rate of 21.2 ml·min−1·gcat−1. The reaction at 295 K produced 12.7 ml·min−1·gcat−1 of hydrogen gas, which is significantly lower than the 303 K, but significantly higher than the 2.8 ml·min−1·gcat−1 of the 273 K condition (Fig. 10). From this data, the activation energy of the reaction as catalyzed by the PtMWCNTs composite can be calculated using an Arrhenius plot (Fig. 11) and the corresponding equation (Eq. 2).
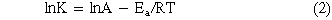
This calculation resulted in an activation energy of 46.2 kJ mol−1, which compares favorably to many catalysts other of this reaction (Table I) but is slightly higher than similar platinum-based catalysts or previous studies using gold nanoparticles. This is believed to be caused by the highly dispersed small (∼3 nm) Platinum Nanoparticles over Carbon Nanotubes.
Figure 9. Comparison of the volume of hydrogen gas vs time generated by the hydrolysis reaction of sodium borohydride as catalyzed by the produced Pt composite under the pH conditions (pH 6, 7, 8).
Download figure:
Standard image High-resolution imageFigure 10. Comparison of the volume of hydrogen gas vs time generated by the hydrolysis reaction of sodium borohydride as catalyzed by the produced platinum composite at 273 K, 295 K, and 303 K.
Download figure:
Standard image High-resolution imageTable I. Reported activation energies for NaBH4 hydrolysis by catalyst.
| Catalyst | Ea (kJ mol−1) | Temperature (K) | Time (min) | References |
|---|---|---|---|---|
| Pd/C | 28.0 | 283–328 | 20 | 37 |
| Ru/Graphite | 61.1 | 298–318 | 30 | 40 |
| Pt-Pd-CNTs | 19.0 | 302–332 | 120 | 38 |
| MWCNT supported Co | 40.40 | 298–333 | 20 | 39 |
| Raney Co | 53.7 | 283–303 | 50 | 5 |
| Co Nanoparticles | 35.0 | 313–353 | 10 | 41 |
| Pt/CeO2-Co7Ni2Ox | 47.4 | 298–318 | 13 | 42 |
| CuNWs | 42.48 | 298–333 | 9–250 | 43 |
| NaBH4@Ni | 46.6 | 283–333 | 10 | 44 |
| Co/Fe3O4@C | 49.2 | 288–328 | 30 | 45 |
| Cu based catalysts | 61.16 | 293–313 | 40–130 | 46 |
| Ni-Co-P/γ-Al2O3 | 52.05 | 298–328 | 35 | 47 |
| AuMWCNTs | 21.1 | 273–303 | 120 | 21 |
| AgMWCNTs | 44.5 | 273–303 | 120 | 22 |
| PdMWCNTs | 62.6 | 273–303 | 120 | 23 |
| PtMWCNTs | 46.2 | 273–303 | 120 | This Work |
Figure 11. Arrhenius Plot for determining the activation energy of the reaction as catalyzed by the PtMWCNTs composite.
Download figure:
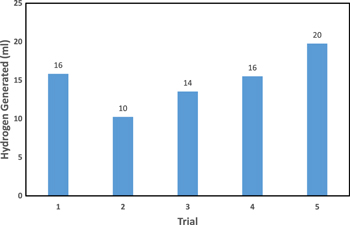
Standard image High-resolution imageFigure 12. Catalytic Reusability of PtMWCNTs.
Download figure:
Standard image High-resolution imageThe PtMWCNTs composite displayed its potential durability through the reusability trial which lasts for 10 h (Fig. 12). The amount of hydrogen generation appears to be slightly decreased after the first trial, but it steadily increased and showed it catalytic outperformance in the fifth trial. The reusability trial produced an average of 15.2 ml of hydrogen. These results highly suggested that the material is stable and has a high recycle potential for hydrogen economy.
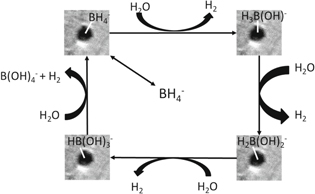
The proposed mechanism of the catalyzed hydrolysis of sodium borohydride by PtMWCNTs is presented in Scheme
Scheme 1. Proposed mechanism for hydrolysis of sodium borohydride catalyzed by PtMWCNTs composite.
Download figure:
Standard image High-resolution imageConclusions
The PtMWCNTs composite catalyst was successfully synthesized and characterized by P-XRD, FTIR, SEM-EDS and TEM. The composite outperformed the original platinum nanoparticles. This is due to highly dispersed platinum nanoparticles over MWCNTs. The composite performed best at pH 8 implying a sensitivity of pH for the composite catalyst as sodium borohydride is known to react faster at a lower pH. Temperature adjusted trials allowed for the calculation of activation energy, which compares favorably to other reported catalysts at 46.2 kJ mol−1 but did not outperform previous works with AuMWCNTs composites that had higher loading of noble metal nanoparticles. This further implies the importance of the concentration of metal nanoparticles within the composite. Overall, the PtMWCNTs composite shows promise as a durable catalyst for the hydrolysis of aqueous sodium borohydride.
Acknowledgments
Corresponding Author acknowledge Dr. Lawrence J. Sacks Professorship in Chemistry.