Selective Removal of As(V) Ions from Acid Mine Drainage Using Polymer Inclusion Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
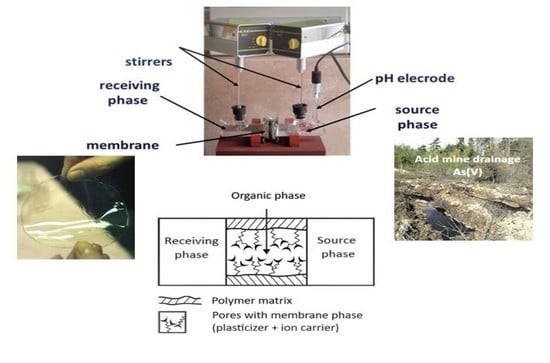
2.2. Preparation Of Polymer Inclusion Membranes and Stability Test
2.3. Transport Studies
3. Results and Discussion
3.1. Kinetics of As(V) Ions Transport through Polymer Inclusion Membranes
3.2. Effect of Membrane Composition
3.3. Modification of the Source Phase Acidity
3.4. Membrane Reusability
3.5. Selective Removal of As(V) from AMD
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Akcil, A.; Koldas, S. Acid Mine Drainage (AMD): Causes, treatment and case studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Othman, A.; Sulaiman, A.; Ibrahim, I. The use of low grade limestone in acid mine drainage treatment. Malays. J. Anal. Sci. 2019, 23, 472–478. [Google Scholar]
- Burillo, J.C.; Castro-Larragoitia, J.; Burillo, G.; Ortega, A.; Medellin-Castillo, N. Removal of arsenic and iron from mine-tailing leachate using chitosan hydrogels synthesized by gamma radiation. Environ. Earth Sci. 2017, 76, 450. [Google Scholar] [CrossRef]
- Serrano, J.; Leiva, E. Removal of arsenic using acid/metal-tolerant sulfate reducing bacteria: A new approach for bioremediation of high-arsenic acid mine waters. Water 2017, 9, 994. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.-J. Biosorption of heavy metals from acid mine drainage by modified sericite and microalgae hybrid system. Water Air Soil Pollut. 2015, 226, 185. [Google Scholar] [CrossRef]
- Kato, T.; Kawasaki, Y.; Kadokura, M.; Suzuki, K.; Tawara, Y.; Ohara, Y.; Tokoro, C. Application of GETFLOWS coupled with chemical reactions to arsenic removal through ferrihydrite coprecipitation in an artificial wetland of a Japanese closed mine. Minerals 2020, 10, 475. [Google Scholar] [CrossRef]
- Skousen, J.; Zipper, C.E.; Rose, A.; Ziemkiewicz, P.F.; Nairn, R.; McDonald, L.M.; Kleinmann, R.L. Review of passive systems for acid mine drainage treatment. Mine Water Environ. 2017, 36, 133–153. [Google Scholar] [CrossRef] [Green Version]
- Luo, C.; Routh, J.; Dario, M.; Sarkar, S.; Wei, L.; Luo, D.; Liu, Y. Distribution and mobilization of heavy metals at an acid mine drainage affected region in South China, a post-remediation study. Sci. Total Environ. 2020, 724, 138122. [Google Scholar] [CrossRef]
- Obiri-Nyarko, F.; Kwiatkowska-Malina, J.; Malina, G.; Wołowiec, K. Assessment of zeolite and compost-zeolite mixture as permeable reactive materials for the removal of lead from a model acidic groundwater. J. Contam. Hydrol. 2020, 229, 103597. [Google Scholar] [CrossRef]
- Kjoller, C.; Postma, D.; Larsen, F. Groundwater acidification and the mobilization of trace metals in a sandy aquifer. Environ. Sci. Technol. 2004, 38, 2829–2835. [Google Scholar] [CrossRef]
- Simate, G.S.; Ndlovu, S. Acid mine drainage: Challenges and opportunities. J. Environ. Chem. Eng. 2014, 2, 1785–1803. [Google Scholar] [CrossRef]
- Al-Abed, S.; Jegadeesan, G.; Purandare, J.; Allen, D. Arsenic release from iron rich mineral processing waste: Influence of pH and redox potential. Chemosphere 2007, 66, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Tokoro, C.; Kadokura, M.; Kato, T. Mechanism of arsenate coprecipitation at the solid/liquid interface of ferrihydrite: A perspective review. Adv. Powder Technol. 2020, 31, 859–866. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef]
- Malina, G. Ecotoxicological and environmental problems associated with the former chemical plant in Tarnowskie Góry, Poland. Toxicology 2004, 205, 157–172. [Google Scholar] [CrossRef]
- Pous, N.; Casentini, B.; Rossetti, S.; Fazi, S.; Puig, S.; Aulenta, F. Anaerobic arsenite oxidation with an electrode serving as the sole electron acceptor: A novel approach to the bioremediation of arsenic-polluted groundwater. J. Hazard. Mater. 2015, 283, 617–622. [Google Scholar] [CrossRef]
- WHO 2011. Arsenic in Drinking Water; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Henke, K.; Hutchison, A. Arsenic Chemistry. In Arsenic: Environmental Chemistry, Health Threats and Waste Treatment; Henke, K.R., Ed.; John Wiley & Sons Ltd.: West Sussex, UK, 2009; pp. 154–196. [Google Scholar]
- Majzlan, J.; Plášil, J.; Škoda, R.; Gescher, J.; Kögler, F.; Rusznyak, A.; Küsel, K.; Neu, T.R.; Mangold, S.; Rothe, J. Arsenic-rich acid mine water with extreme arsenic concentration: Mineralogy, geochemistry, microbiology, and environmental implications. Environ. Sci. Technol. 2014, 48, 13685–13693. [Google Scholar] [CrossRef]
- Tardy, V.; Casiot, C.; Fernandez-Rojo, L.; Resongles, E.; Desoeuvre, A.; Joulian, C.; Battaglia-Brunet, F.; Héry, M. Temperature and nutrients as drivers of microbially mediated arsenic oxidation and removal from acid mine drainage. Appl. Microbiol. Biotechnol. 2018, 102, 2413–2424. [Google Scholar] [CrossRef]
- Abdul, K.S.M.; Jayasinghe, S.S.; Chandana, E.P.S.; Jayasumana, C.; Silva, P.M.C.S.D. Arsenic and human health effects: A review. Environ. Toxicol. Pharmacol. 2015, 40, 828–846. [Google Scholar] [CrossRef]
- Abrosimova, N.A.; Saeva, O.P.; Bortnikova, S.B.; Edelev, A.V.; Korneeva, T.V.; Yurkevich, N.V. Metals and metalloids removal from mine water using natural and modified heulandite. Int. J. Environ. Sci. Dev. 2019, 10, 202–205. [Google Scholar] [CrossRef]
- Cui, M.; Jang, M.; Cho, S.-H.; Khim, J.; Cannon, F.S. A continuous pilot-scale system using coal-mine drainage sludge to treat acid mine drainage contaminated with high concentrations of Pb, Zn, and other heavy metals. J. Hazard. Mater. 2012, 215–216, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Nicomel, N.R.; Leus, K.; Folens, K.; Van Der Voort, P.; Du Laing, G. Technologies for arsenic removal from water: Current status and future perspectives. Int. J. Environ. Res. Public Health 2016, 13, 62. [Google Scholar] [CrossRef]
- Mondal, P.; Bhowmick, S.; Chatterjee, D.; Figoli, A.; Van der Bruggen, B. Remediation of inorganic arsenic in groundwater for safe water supply: A critical assessment of technological solutions. Chemosphere 2013, 92, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Hristovski, K.; Baumgardner, A.; Westerhoff, P. Selecting metal oxide nanomaterials for arsenic removal in fixed bed columns: From nanopowders to aggregated nanoparticle media. J. Hazard. Mater. 2007, 147, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, X. On the potential of biological treatment for arsenic contaminated soils and groundwater. J. Environ. Manag. 2009, 90, 2367–2376. [Google Scholar] [CrossRef]
- Zagury, G.J.; Kulnieks, V.I.; Neculita, C.M. Characterization and reactivity assessment of organic substrates for sulphate-reducing bacteria in acid mine drainage treatment. Chemosphere 2006, 64, 944–954. [Google Scholar] [CrossRef]
- Costello, C. Acid Mine Drainage: Innovative Treatment Technologies; U.S. EPA Office of Solid Waste and Emergency Response Technology Innovation Office: Washington, DC, USA, 2003. [Google Scholar]
- Blowes, D.W.; Ptacek, C.J.; Benner, S.G.; McRae, C.W.T.; Bennett, T.A.; Puls, R. Treatment of inorganic contaminants using permeable reactive barriers. J. Contam. Hydrol. 2000, 45, 123–137. [Google Scholar] [CrossRef]
- Zawierucha, I.; Nowik-Zajac, A.; Kozlowski, C. Removal of Pb(II) ions using polymer inclusion membranes containing calix[4]resorcinarene derivative as ion carrier. Polymers 2019, 11, 2111. [Google Scholar] [CrossRef] [Green Version]
- Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Recent trends in extraction and transport of metal ions using polymer inclusion membranes (PIMs). J. Membr. Sci. 2012, 415–416, 9–23. [Google Scholar] [CrossRef]
- Cho, Y.; Cattrall, R.W.; Kolev, S.D. A novel polymer inclusion membrane based method for continuous clean-up of thiocyanate from gold mine tailings water. J. Hazard. Mater. 2018, 341, 297–303. [Google Scholar] [CrossRef]
- Wieszczycka, K.; Staszak, K. Polymers in separation processes. In Polymer Engineering, 1st ed.; Tylkowski, B., Wieszczycka, K., Jastrzab, R., Eds.; De Gruyter: Poznan, Poland, 2017; pp. 235–276. [Google Scholar]
- Zawierucha, I.; Nowik-Zajac, A.; Kozlowski, C. Application of Cr(VI) transport across the polymer inclusion membrane with calixresorcin[4]arene derivative as ion carrier. Sep. Sci. Technol. 2020, 55, 2204–2210. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Mornane, P.; Potter, I.D.; Perera, J.M.; Cattrall, R.W.; Kolev, S.D. Extraction and transport of metal ions and small organic compounds using polymer inclusion membranes (PIMs). J. Membr. Sci. 2006, 281, 7–41. [Google Scholar] [CrossRef]
- Zawierucha, I.; Kozlowski, C.; Malina, G. Immobilized materials for removal of toxic metal ions from surface/groundwaters and aqueous waste streams. Environ. Sci. Proc. Impacts 2016, 18, 429–444. [Google Scholar] [CrossRef]
- Guell, R.; Anticó, E.; Kolev, S.D.; Benavente, J.; Salvadó, V.; Fontàs, C. Development and characterization of polymer inclusion membranes for the separation and speciation of inorganic As species. J. Membr. Sci. 2011, 383, 88–95. [Google Scholar] [CrossRef]
- Vera, R.; Antico, E.; Fontas, C. The use of a polymer inclusion membrane for arsenate determination in groundwater. Water 2018, 10, 1093. [Google Scholar] [CrossRef] [Green Version]
- De Lourdes Ballinas, M.; De San Miguel, E.R.; De Jesus Rodriguez, M.T.; Silva, O.; Munoz, M.; De Gyves, J. Arsenic(V) removal with polymer inclusion membranes from sulfuric acid media using DBBP as carrier. Environ. Sci. Technol. 2004, 38, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Danesi, P.R. Separation of metal species by supported liquid membranes. Sep. Sci. Technol. 1984, 19, 857–894. [Google Scholar] [CrossRef]
- Nowik-Zajac, A.; Zawierucha, I.; Kozlowski, C. Selective removal of silver(I) using polymer inclusion membranes containing calixpyrolles. RSC Adv. 2019, 9, 31122–31132. [Google Scholar] [CrossRef] [Green Version]
- Pospiech, B.; Kujawski, W. Ionic liquids as selective extractants and ion carriers of heavy metal ions from aqueous solutions utilized in extraction and membrane separation. Rev. Chem. Eng. 2015, 31, 179–191. [Google Scholar] [CrossRef]
- Marino, T.; Figoli, A. Arsenic removal by liquid membranes. Membranes 2015, 5, 150–167. [Google Scholar] [CrossRef] [Green Version]
- Alguacil, F.J.; López-Delgado, A.; Alonso, M.; Sastre, A.M. The phosphine oxides Cyanex 921 and Cyanex 923 as carriers for facilitated transport of chromium (VI)-chloride aqueous solutions. Chemosphere 2004, 57, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Kaya, A.; Alpoguz, H.K.; Yilmaz, A. Application of Cr(VI) transport through the polymer inclusion membrane with a new synthesized calix[4]arene derivative. Ind. Eng. Chem. Res. 2013, 52, 5428–5436. [Google Scholar] [CrossRef]
- Martinez Perez, M.E.; Reyes-Aguilera, J.A.; Saucedo, T.I.; Gonzalez, M.P.; Navarro, R.; Avila-Rodriguez, M. Study of As(V) transfer through a supported liquid membrane impregnated with trioctylphosphine oxide (Cyanex 921). J. Membr. Sci. 2007, 302, 119–126. [Google Scholar] [CrossRef]
- US Environmental Protection Agency (EPA). Method 8061A: Phthalate Esters by Gas Chromatography with Electron Capture Detection (GC/ECD); US Environmental Protection Agency: Cincinnati, OH, USA, 1996.
- Chen, X.; Xu, S.; Tan, T.; Lee, S.T.; Cheng, S.H.; Lee, F.W.F.; Xu, S.J.L.; Ho, K.C. Toxicity and estrogenic endocrine disrupting activity of phthalates and their mixtures. Int. J. Environ. Res. Public Health 2014, 11, 3156–3168. [Google Scholar] [CrossRef] [PubMed]
- Iberhan, L.; Wisniewski, M. Extraction of arsenic(III) and arsenic(V) with Cyanex 925, Cyanex 301 and their mixtures. Hydrometallurgy 2002, 63, 23–30. [Google Scholar] [CrossRef]
- Migaszewski, Z.M.; Gałuszka, A.; Dołegowska, S. Extreme enrichment of arsenic and rare earth elements in acid mine drainage: Case study of Wisniowka mining area (south-central Poland). Environ. Pollut. 2019, 244, 898–906. [Google Scholar] [CrossRef]
- Ruehl, M.D.; Hiibel, S.R. Evaluation of organic carbon and microbial inoculum for bioremediation of acid mine drainage. Miner. Eng. 2020, 157, 106554. [Google Scholar] [CrossRef]
- Costa, M.C.; Duarte, J.C. Bioremediation of acid mine drainage using acidic soil and organic wastes for promoting sulphate-reducing bacteria activity on a column reactor. Water Air Soil Pollut. 2005, 165, 325–345. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Verma, V.; Tewari, S.; Rai, J. Ion exchange during heavy metal bio-sorption from aqueous solution by dried biomass of macrophytes. Bioresour. Technol. 2008, 99, 1932–1938. [Google Scholar] [CrossRef]
- Cochrane, E.; Lu, S.; Gibb, S.; Villaescusa, I.; Gibb, S. A comparison of low-cost biosorbents and commercial sorbents for the removal of copper from aqueous media. J. Hazard. Mater. 2006, 137, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Landaburu-Aguirre, J.; Pongracz, E.; Peramaki, P.; Keiski, R.L. Micellar-enhanced ultrafiltration for the removal of cadmium and zinc: Use of response surface methodology to improve understanding of process performance and optimisation. J. Hazard. Mater. 2010, 180, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Amy, G.; Chung, J.; Sohn, J.; Yoon, Y. Removal of toxic ions (chromate, arsenate, and perchlorate) using reverse osmosis, nanofiltration, and ultrafiltration membranes. Chemosphere 2009, 77, 228–235. [Google Scholar] [CrossRef]
- Lertlapwasin, R.; Bhawawet, N.; Imyim, A.; Fuangswasdi, S. Ionic liquid extraction of heavy metal ions by 2-aminothiophenol in 1-butyl-3-methylimidazolium hexafluorophosphate and their association constants. Sep. Purif. Technol. 2010, 72, 70–76. [Google Scholar] [CrossRef]
- Akbal, F.; Camci, S. Copper, chromium and nickel removal from metal plating wastewater by electrocoagulation. Desalination 2011, 269, 214–222. [Google Scholar] [CrossRef]
- Pohl, A. Removal of heavy metal ions from water and wastewaters by sulfur-containing precipitation agents. Water Air Soil Pollut. 2020, 231, 503. [Google Scholar] [CrossRef]
- Qdais, H.A.; Moussa, H. Removal of heavy metals from wastewater by membrane processes: A comparative study. Desalination 2004, 164, 105–110. [Google Scholar] [CrossRef]
- Ruparelia, J.P.; Duttagupta, S.P.; Chatterjee, A.K.; Mukherji, S. Potential of carbon nanomaterials for removal of heavy metals from water. Desalination 2008, 232, 145–156. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Z.; Jiang, G. Coating Fe3O4 magnetic nanoparticles with humic acid for high efficient removal of heavy metals in water. Environ. Sci. Technol. 2008, 42, 6949–6954. [Google Scholar] [CrossRef] [PubMed]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zawierucha, I.; Nowik-Zajac, A.; Malina, G. Selective Removal of As(V) Ions from Acid Mine Drainage Using Polymer Inclusion Membranes. Minerals 2020, 10, 909. https://doi.org/10.3390/min10100909
Zawierucha I, Nowik-Zajac A, Malina G. Selective Removal of As(V) Ions from Acid Mine Drainage Using Polymer Inclusion Membranes. Minerals. 2020; 10(10):909. https://doi.org/10.3390/min10100909
Chicago/Turabian StyleZawierucha, Iwona, Anna Nowik-Zajac, and Grzegorz Malina. 2020. "Selective Removal of As(V) Ions from Acid Mine Drainage Using Polymer Inclusion Membranes" Minerals 10, no. 10: 909. https://doi.org/10.3390/min10100909