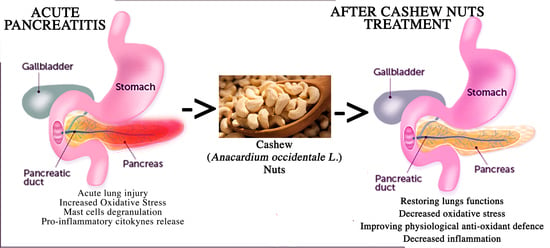
Cashew (Anacardium occidentale L.) Nuts Modulate the Nrf2 and NLRP3 Pathways in Pancreas and Lung after Induction of Acute Pancreatitis by Cerulein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Protocol
2.3. Experimental Groups
- (1)
- Sham: Animals were subjected to injections of saline and were treated by oral gavage with saline.
- (2)
- Sham + cashew nuts (100 mg/kg): Animals were subjected to injections of saline and were treated by oral gavage with cashew nuts at the dose of 100 mg/kg (data not shown because there were no differences between the sham+saline and sham+cashew nuts groups.).
- (3)
- Cerulein: Mice were subjected to cerulein injections as described above and treated by oral gavage with saline.
- (4)
- Cashew nuts (100 mg/kg): Mice were subjected to cerulein injections a described above and treated by oral gavage with cashew nuts (100 mg/kg).
2.4. Pancreatic and Lung Oedema
2.5. Histological Evaluation and Detection of Mast Cells
2.6. Measurement of Lipase, Amylase and Pro-Inflammatory Citokynes
2.7. Evaluation of Myeloperoxidas and Malonaldehyde
2.8. Western Blot Analysis
2.9. Immunohistochemical Localization of NRF2, HO-1, Mn-SOD, Caspase-1, and ASC
2.10. Cashew Nuts Nutritional Composition
2.11. Reagents
2.12. Data Analysis
3. Results
3.1. Effect of Cashew Nuts on Cerulein-Induced Oedema and Tissue Damage
3.2. Effects of Cashew Nuts on Cerulein-Induced Mast Cell Degranulation and on Myeloperoxidase and Malondialdehyde Activity
3.3. Effects of Cashew Nuts on the Levels of Amylase, Lipase, and Pro-Inflammatory Cytokines
3.4. Effects of Cashew Nuts on the Nrf2 Pathway in Cerulein-Induced AP
3.5. Effects of Cashew Nuts on the NLRP3 Pathway in Cerulein-Induced AP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, Z.S.; Ku, C.F.; Guan, Y.F.; Xiao, H.T.; Shi, X.K.; Wang, H.Q.; Bian, Z.X.; Tsang, S.W.; Zhang, H.J. Dihydro-Resveratrol Ameliorates Lung Injury in Rats with Cerulein-Induced Acute Pancreatitis. Phytother. Res. 2016, 30, 663–670. [Google Scholar] [CrossRef]
- Charbonney, E.; Nathens, A.B. Severe acute pancreatitis: A review. Surg. Infect. 2008, 9, 573–578. [Google Scholar] [CrossRef] [Green Version]
- Bansal, A.; Gupta, P.; Singh, H.; Samanta, J.; Mandavdhare, H.; Sharma, V.; Sinha, S.K.; Dutta, U.; Kochhar, R. Gastrointestinal complications in acute and chronic pancreatitis. JGH Open 2019, 3, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Raraty, M.G.; Connor, S.; Criddle, D.N.; Sutton, R.; Neoptolemos, J.P. Acute pancreatitis and organ failure: Pathophysiology, natural history, and management strategies. Curr. Gastroenterol. Rep. 2004, 6, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, F.; Gul, M.; Esrefoglu, M.; Ates, B. The contradictory effects of nitric oxide in caerulein-induced acute pancreatitis in rats. Free Radic Res. 2008, 42, 289–296. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, F.; Yang, L.; Zou, J.; Wang, H.; Liu, K.; Liu, M.; Zhang, H.; Xiao, X.; Wang, K. Resveratrol protects against L-arginine-induced acute necrotizing pancreatitis in mice by enhancing SIRT1-mediated deacetylation of p53 and heat shock factor 1. Int. J. Mol. Med. 2017, 40, 427–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Q.H.; Zhang, P.X.; Liu, Y.; Liu, W.; Yin, N. Hyperbaric oxygen preconditioning protects the lung against acute pancreatitis induced injury via attenuating inflammation and oxidative stress in a nitric oxide dependent manner. Biochem. Biophys Res. Commun. 2016, 478, 93–100. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.D.; Yu, J.; Chi, J.L.; Long, F.W.; Yang, H.W.; Chen, K.L.; Lv, Z.Y.; Zhou, B.; Peng, Z.H.; et al. Deletion Of XIAP reduces the severity of acute pancreatitis via regulation of cell death and nuclear factor-kappaB activity. Cell Death Dis. 2017, 8, e2685. [Google Scholar] [CrossRef]
- Seo, S.W.; Bae, G.S.; Kim, S.G.; Yun, S.W.; Kim, M.S.; Yun, K.J.; Park, R.K.; Song, H.J.; Park, S.J. Protective effects of Curcuma longa against cerulein-induced acute pancreatitis and pancreatitis-associated lung injury. Int. J. Mol. Med. 2011, 27, 53–61. [Google Scholar] [CrossRef]
- Aruna, R.; Geetha, A.; Suguna, P. Rutin modulates ASC expression in NLRP3 inflammasome: A study in alcohol and cerulein-induced rat model of pancreatitis. Mol. Cell Biochem. 2014, 396, 269–280. [Google Scholar] [CrossRef]
- Hoque, R.; Sohail, M.; Malik, A.; Sarwar, S.; Luo, Y.; Shah, A.; Barrat, F.; Flavell, R.; Gorelick, F.; Husain, S.; et al. TLR9 and the NLRP3 inflammasome link acinar cell death with inflammation in acute pancreatitis. Gastroenterology 2011, 141, 358–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, X.; Li, P.; Yin, W.; Ma, L.; Zhang, B.; Zhen, L.; Meng, Y.; Han, L.; Wang, Y. Overexpression of Nrf2 Protects Against Lipopolysaccharide and Cerulein-Induced Pancreatitis In Vitro and In Vivo. Pancreas 2020, 49, 420–428. [Google Scholar] [CrossRef] [PubMed]
- York, J.M.; Castellanos, K.J.; Cabay, R.J.; Fantuzzi, G. Inhibition of the nucleotide-binding domain, leucine-rich containing family, pyrin-domain containing 3 inflammasome reduces the severity of experimentally induced acute pancreatitis in obese mice. Transl. Res. 2014, 164, 259–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Z.; Shang, H.; Chen, Y.Q.; Pan, L.L.; Bhatia, M.; Sun, J. Sulforaphane Protects Pancreatic Acinar Cell Injury by Modulating Nrf2-Mediated Oxidative Stress and NLRP3 Inflammatory Pathway. Oxid. Med. Cell. Longev. 2016, 2016, 7864150. [Google Scholar] [CrossRef] [PubMed]
- Hybertson, B.M.; Gao, B.; Bose, S.K.; McCord, J.M. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Mol. Aspects Med. 2011, 32, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Bourliere, M.; Rabiega, P.; Ganne-Carrie, N.; Serfaty, L.; Marcellin, P.; Pouget, N.; Guyader, D.; Hezode, C.; Picon, M.; Causse, X.; et al. HBsAg Clearance after addition of 48 weeks of pegifn in hbeag negative chb patients on nucleos(t)ide therapy with undetectable hbvdna for at least one year: Final results from anrs-hb06 pegan study: Multicenter randomized controlled phase iii trial. J. Hepatol. 2015, 62, S249. [Google Scholar] [CrossRef]
- Wang, M.X.; Zhao, J.; Zhang, H.; Li, K.; Niu, L.Z.; Wang, Y.P.; Zheng, Y.J. Potential Protective and Therapeutic Roles of the Nrf2 Pathway in Ocular Diseases: An Update. Oxid. Med. Cell. Longev. 2020, 2020, 9410952. [Google Scholar] [CrossRef]
- Robledinos-Anton, N.; Fernandez-Gines, R.; Manda, G.; Cuadrado, A. Activators and Inhibitors of NRF2: A Review of Their Potential for Clinical Development. Oxid. Med. Cell. Longev. 2019, 2019, 9372182. [Google Scholar] [CrossRef]
- Maes, M.; Fisar, Z.; Medina, M.; Scapagnini, G.; Nowak, G.; Berk, M. New drug targets in depression: Inflammatory, cell-mediated immune, oxidative and nitrosative stress, mitochondrial, antioxidant, and neuroprogressive pathways. And new drug candidates--Nrf2 activators and GSK-3 inhibitors. Inflammopharmacology 2012, 20, 127–150. [Google Scholar] [CrossRef]
- Fusco, R.; Siracusa, R.; Genovese, T.; Cuzzocrea, S.; Di Paola, R. Focus on the Role of NLRP3 Inflammasome in Diseases. Int. J. Mol. Sci. 2020, 21, 4223. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019, 10, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Gao, Q.; Wang, T.; Kan, Z.; Li, X.; Hu, L.; Peng, C.Y.; Qian, F.; Wang, Y.; Granato, D. Green tea polyphenols and epigallocatechin-3-gallate protect against perfluorodecanoic acid induced liver damage and inflammation in mice by inhibiting NLRP3 inflammasome activation. Food Res. Int. 2020, 127, 108628. [Google Scholar] [CrossRef] [PubMed]
- Tozser, J.; Benko, S. Natural Compounds as Regulators of NLRP3 Inflammasome-Mediated IL-1beta Production. Mediators Inflamm. 2016, 2016, 5460302. [Google Scholar] [CrossRef] [Green Version]
- Agila, A.; Barringer, S.A. Volatile profile of cashews (Anacardium occidentale L.) from different geographical origins during roasting. J. Food Sci. 2011, 76, C768–C774. [Google Scholar] [CrossRef]
- de Melo, M.; Pereira, D.E.; Sousa, M.M.; Medeiros, D.M.F.; Lemos, L.T.M.; Madruga, M.S.; Santos, N.M.; de Oliveira, M.E.G.; de Menezes, C.C.; Soares, J.K.B. Maternal intake of cashew nuts accelerates reflex maturation and facilitates memory in the offspring. Int. J. Dev. Neurosci. 2017, 61, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Baptista, A.; Goncalves, R.V.; Bressan, J.; Peluzio, M. Antioxidant and Antimicrobial Activities of Crude Extracts and Fractions of Cashew (Anacardium occidentale L.), Cajui (Anacardium microcarpum), and Pequi (Caryocar brasiliense C.): A Systematic Review. Oxid. Med. Cell. Longev. 2018, 2018, 3753562. [Google Scholar] [CrossRef] [Green Version]
- Alexiadou, K.; Katsilambros, N. Nuts: Anti-atherogenic food? Eur. J. Intern. Med. 2011, 22, 141–146. [Google Scholar] [CrossRef]
- Gomez-Caravaca, A.M.; Verardo, V.; Caboni, M.F. Chromatographic techniques for the determination of alkyl-phenols, tocopherols and other minor polar compounds in raw and roasted cold pressed cashew nut oils. J. Chromatogr. A 2010, 1217, 7411–7417. [Google Scholar] [CrossRef]
- Liu, C.M.; Peng, Q.; Zhong, J.Z.; Liu, W.; Zhong, Y.J.; Wang, F. Molecular and Functional Properties of Protein Fractions and Isolate from Cashew Nut (Anacardium occidentale L.). Molecules 2018, 23, 393. [Google Scholar] [CrossRef] [Green Version]
- Siracusa, R.; Fusco, R.; Peritore, A.F.; Cordaro, M.; D’Amico, R.; Genovese, T.; Gugliandolo, E.; Crupi, R.; Smeriglio, A.; Mandalari, G.; et al. The Antioxidant and Anti-Inflammatory Properties of Anacardium occidentale L. Cashew Nuts in a Mouse Model of Colitis. Nutrients 2020, 12, 834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fusco, R.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; D’Amico, R.; Cordaro, M.; Crupi, R.; Mandalari, G.; Impellizzeri, D.; et al. The Role of Cashew (Anacardium occidentale L.) Nuts on an Experimental Model of Painful Degenerative Joint Disease. Antioxidants 2020, 9, 511. [Google Scholar] [CrossRef]
- Batista, K.S.; Alves, A.F.; Lima, M.D.S.; da Silva, L.A.; Lins, P.P.; de Sousa Gomes, J.A.; Silva, A.S.; Toscano, L.T.; de Albuquerque Meireles, B.R.L.; de Magalhaes Cordeiro, A.M.T.; et al. Beneficial effects of consumption of acerola, cashew or guava processing by-products on intestinal health and lipid metabolism in dyslipidaemic female Wistar rats. Br. J. Nutr. 2018, 119, 30–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, C.C.Q.; Madruga, M.S.; Pintado, M.M.E.; Almeida, G.H.O.; Alves, A.P.V.; Dantas, F.A.; Bezerra, J.K.G.; de Melo, M.; Viera, V.B.; Soares, J.K.B. Cashew nuts (Anacardium occidentale L.) decrease visceral fat, yet augment glucose in dyslipidemic rats. PLoS ONE 2019, 14, e0225736. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, A.S.; Nascimento, J.R.; Trovao, L.O.; Alves, P.C.S.; Maciel, M.C.G.; Silva, L.D.M.; Marques, A.A.; Santos, A.; Silva, L.A.; Nascimento, F.R.F.; et al. The anti-inflammatory activity of Anacardium occidentale L. increases the lifespan of diabetic mice with lethal sepsis. J Ethnopharmacol 2019, 236, 345–353. [Google Scholar] [CrossRef]
- Cordaro, M.; Siracusa, R.; Fusco, R.; D’Amico, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; Scuto, M.; Crupi, R.; Mandalari, G.; et al. Cashew (Anacardium occidentale L.) Nuts Counteract Oxidative Stress and Inflammation in an Acute Experimental Model of Carrageenan-Induced Paw Edema. Antioxidants 2020, 9, 660. [Google Scholar] [CrossRef]
- Gebhardt, A.; Ackermann, W.; Unver, N.; Elsasser, H.P. Expression of galectin-3 in the rat pancreas during regeneration following hormone-induced pancreatitis. Cell Tissue Res 2004, 315, 321–329. [Google Scholar] [CrossRef]
- Kim, H. Cerulein pancreatitis: Oxidative stress, inflammation, and apoptosis. Gut Liver 2008, 2, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Fang, X.; Wang, F.; Li, H.; Niu, W.; Liang, W.; Wu, C.; Li, J.; Tu, X.; Pan, L.L.; et al. Butyrate ameliorates caerulein-induced acute pancreatitis and associated intestinal injury by tissue-specific mechanisms. Br. J. Pharmacol. 2019, 176, 4446–4461. [Google Scholar] [CrossRef] [Green Version]
- Ye, W.; Zheng, C.; Yu, D.; Zhang, F.; Pan, R.; Ni, X.; Shi, Z.; Zhang, Z.; Xiang, Y.; Sun, H.; et al. Lipoxin A4 Ameliorates Acute Pancreatitis-Associated Acute Lung Injury through the Antioxidative and Anti-Inflammatory Effects of the Nrf2 Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 2197017. [Google Scholar] [CrossRef] [Green Version]
- Fusco, R.; Cordaro, M.; Siracusa, R.; D’Amico, R.; Genovese, T.; Gugliandolo, E.; Peritore, A.F.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. Biochemical Evaluation of the Antioxidant Effects of Hydroxytyrosol on Pancreatitis-Associated Gut Injury. Antioxidants 2020, 9, 781. [Google Scholar] [CrossRef]
- Heindl, M.; Tuennemann, J.; Sommerer, I.; Mossner, J.; Hoffmeister, A. Loss of Bace1 in mice does not alter the severity of caerulein induced pancreatitis. PLoS ONE 2015, 10, e0125556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, C.; Song, Q.; Wang, P.; Li, Y.; Yang, M.; Yu, S.Y. Neuroprotective Effects of Curcumin on IL-1beta-Induced Neuronal Apoptosis and Depression-Like Behaviors Caused by Chronic Stress in Rats. Front. Cell. Neurosci. 2018, 12, 516. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Mazzon, E.; Esposito, E.; Muia, C.; Abdelrahman, M.; Di Paola, R.; Crisafulli, C.; Bramanti, P.; Thiemermann, C. Glycogen synthase kinase-3beta inhibition attenuates the development of ischaemia/reperfusion injury of the gut. Intensive Care Med. 2007, 33, 880–893. [Google Scholar] [CrossRef]
- Costantino, G.; Cuzzocrea, S.; Mazzon, E.; Caputi, A.P. Protective effects of melatonin in zymosan-activated plasma-induced paw inflammation. Eur. J. Pharmacol. 1998, 363, 57–63. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Esposito, E.; Di Paola, R.; Ahmad, A.; Campolo, M.; Peli, A.; Morittu, V.M.; Britti, D.; Cuzzocrea, S. Palmitoylethanolamide and luteolin ameliorate development of arthritis caused by injection of collagen type II in mice. Arthritis Res. Ther. 2013, 15, R192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gugliandolo, E.; Fusco, R.; Biundo, F.; D’Amico, R.; Benedetto, F.; Di Paola, R.; Cuzzocrea, S. Palmitoylethanolamide and Polydatin combination reduces inflammation and oxidative stress in vascular injury. Pharmacol. Res. 2017, 123, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Impellizzeri, D.; Gugliandolo, E.; Siracusa, R.; Crupi, R.; Esposito, E.; Cuzzocrea, S. Adelmidrol, a Palmitoylethanolamide Analogue, as a New Pharmacological Treatment for the Management of Inflammatory Bowel Disease. Mol. Pharmacol. 2016, 90, 549–561. [Google Scholar] [CrossRef] [Green Version]
- Sawant, S.; Gokulan, R.; Dongre, H.; Vaidya, M.; Chaukar, D.; Prabhash, K.; Ingle, A.; Joshi, S.; Dange, P.; Joshi, S.; et al. Prognostic role of Oct4, CD44 and c-Myc in radio-chemo-resistant oral cancer patients and their tumourigenic potential in immunodeficient mice. Clin. Oral Investig. 2016, 20, 43–56. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.t.; Smith, F. Colorimetric method for determination of sugars and related substances. Analytical Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Agrawal, N.; Minj, D.K.; Rani, K. Estimation of total carbohydrate present in dry fruits. IOSR J. Environ. Sci. Toxicol. Food Technol. 2015, 1, 24–27. [Google Scholar]
- Smeriglio, A.; Mandalari, G.; Bisignano, C.; Filocamo, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Polyphenolic content and biological properties of Avola almond (Prunus dulcis Mill. DA Webb) skin and its industrial byproducts. Industrial Crops Products 2016, 83, 283–293. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists; Horwitz, W. Official Methods of Analysis. Association of Official Analytical Chemists: Washington, DC, USA, 1975; Volume 222. [Google Scholar]
- Fu, C.Y.; Yeh, C.N.; Hsu, J.T.; Jan, Y.Y.; Hwang, T.L. Timing of mortality in severe acute pancreatitis: Experience from 643 patients. World J. Gastroenterol. 2007, 13, 1966–1969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virlos, I.; Mazzon, E.; Serraino, I.; Di Paola, R.; Genovese, T.; Britti, D.; Thiemerman, C.; Siriwardena, A.; Cuzzocrea, S. Pyrrolidine dithiocarbamate reduces the severity of cerulein-induced murine acute pancreatitis. Shock 2003, 20, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, A.P.; Bernard, G.R. Acute lung injury and the acute respiratory distress syndrome: A clinical review. Lancet 2007, 369, 1553–1564. [Google Scholar] [CrossRef]
- Babu, B.I.; Malleo, G.; Genovese, T.; Mazzon, E.; Di Paola, R.; Crisafulli, C.; Caminiti, R.; Siriwardena, A.K.; Cuzzocrea, S. Green tea polyphenols ameliorate pancreatic injury in cerulein-induced murine acute pancreatitis. Pancreas 2009, 38, 954–967. [Google Scholar] [CrossRef] [PubMed]
- Kuklinski, B.; Zimmermann, T.; Schweder, R. [Decreasing mortality in acute pancreatitis with sodium selenite. Clinical results of 4 years antioxidant therapy]. Med Klin 1995, 90 (Suppl. 1), 36–41. [Google Scholar]
- Du, W.D.; Yuan, Z.R.; Sun, J.; Tang, J.X.; Cheng, A.Q.; Shen, D.M.; Huang, C.J.; Song, X.H.; Yu, X.F.; Zheng, S.B. Therapeutic efficacy of high-dose vitamin C on acute pancreatitis and its potential mechanisms. World J. Gastroenterol. 2003, 9, 2565–2569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolin, I.; Herrera, F.; Martin, V.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar] [CrossRef]
- Shields, C.J.; Winter, D.C.; Redmond, H.P. Lung injury in acute pancreatitis: Mechanisms, prevention, and therapy. Curr. Opin. Crit. Care 2002, 8, 158–163. [Google Scholar] [CrossRef]
- Meagher, E.A.; Barry, O.P.; Lawson, J.A.; Rokach, J.; FitzGerald, G.A. Effects of vitamin E on lipid peroxidation in healthy persons. JAMA 2001, 285, 1178–1182. [Google Scholar] [CrossRef] [PubMed]
- Vincent, H.K.; Bourguignon, C.M.; Vincent, K.R.; Weltman, A.L.; Bryant, M.; Taylor, A.G. Antioxidant supplementation lowers exercise-induced oxidative stress in young overweight adults. Obesity 2006, 14, 2224–2235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogan, S.; Canning, C.; Sun, S.; Sun, X.; Zhou, K. Effects of grape pomace antioxidant extract on oxidative stress and inflammation in diet induced obese mice. J. Agric Food Chem. 2010, 58, 11250–11256. [Google Scholar] [CrossRef]
- Sacchet, C.; Mocelin, R.; Sachett, A.; Bevilaqua, F.; Chitolina, R.; Kuhn, F.; Boligon, A.A.; Athayde, M.L.; Roman Junior, W.A.; Rosemberg, D.B.; et al. Antidepressant-Like and Antioxidant Effects of Plinia trunciflora in Mice. Evid. Based Complement Altern. Med. 2015, 2015, 601503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, I.; Biswas, S.K.; Kirkham, P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 2006, 72, 1439–1452. [Google Scholar] [CrossRef]
- Pereira de Jesus Costa, A.C.; Kelly Dos Santos Silva, M.; Batista de Oliveira, S.; Silva, L.L.; Silva, A.C.; Barroso, R.B.; Macedo Costa, J.R.; Lima Hunaldo, V.K.; Neto, M.S.; Pascoal, L.M.; et al. Effects of Cashew Nut (Anacardium occidentale L.) Seed Flour in Moderately Malnourished Children: Randomized Clinical Trial. J. Nutr. Metab. 2020, 2020, 6980754. [Google Scholar] [CrossRef]
- Melo-Cavalcante, A.A.; Dantas, S.M.; Leite Ade, S.; Matos, L.A.; e Sousa, J.M.; Picada, J.N.; da Silva, J. In vivo antigenotoxic and anticlastogenic effects of fresh and processed cashew (Anacardium occidentale) apple juices. J. Med. Food 2011, 14, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Melo Cavalcante, A.A.; Rubensam, G.; Picada, J.N.; Gomes da Silva, E.; Fonseca Moreira, J.C.; Henriques, J.A. Mutagenicity, antioxidant potential, and antimutagenic activity against hydrogen peroxide of cashew (Anacardium occidentale) apple juice and cajuina. Environ. Mol. Mutagen 2003, 41, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Behravan, E.; Heidari, M.R.; Heidari, M.; Fatemi, G.; Etemad, L.; Taghipour, G.; Abbasifard, M. Comparison of gastric ulcerogenicity of percolated extract of Anacardium occidentale (cashew nut) with indomethacin in rats. Pak J. Pharm. Sci. 2012, 25, 111–115. [Google Scholar]
- Olajide, O.A.; Aderogba, M.A.; Adedapo, A.D.; Makinde, J.M. Effects of Anacardium occidentale stem bark extract on in vivo inflammatory models. J. Ethnopharmacol. 2004, 95, 139–142. [Google Scholar] [CrossRef]
- Carvalho, N.S.; Silva, M.M.; Silva, R.O.; Nicolau, L.A.; Sousa, F.B.; Damasceno, S.R.; Silva, D.A.; Barbosa, A.L.; Leite, J.R.; Medeiros, J.V. Gastroprotective properties of cashew gum, a complex heteropolysaccharide of Anacardium occidentale, in naproxen-induced gastrointestinal damage in rats. Drug Dev. Res. 2015, 76, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Vilar, M.S.; de Souza, G.L.; Vilar Dde, A.; Leite, J.A.; Raffin, F.N.; Barbosa-Filho, J.M.; Nogueira, F.H.; Rodrigues-Mascarenhas, S.; Moura, T.F. Assessment of Phenolic Compounds and Anti-Inflammatory Activity of Ethyl Acetate Phase of Anacardium occidentale L. Bark. Molecules 2016, 21, 1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Silveira Vasconcelos, M.; Gomes-Rochette, N.F.; de Oliveira, M.L.; Nunes-Pinheiro, D.C.; Tome, A.R.; Maia de Sousa, F.Y.; Pinheiro, F.G.; Moura, C.F.; Miranda, M.R.; Mota, E.F.; et al. Anti-inflammatory and wound healing potential of cashew apple juice (Anacardium occidentale L.) in mice. Exp. Biol. Med. 2015, 240, 1648–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, H.B.; Vinayagam, K.S.; Moorthy, B.T.; Palanivelu, S.; Panchanatham, S. Anti-inflammatory and anti-hyperlipidemic effect of Semecarpus anacardium in a high fat diet: STZ-induced type 2 diabetic rat model. Inflammopharmacology 2013, 21, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Font, I.; Gea-Sorli, S.; de-Madaria, E.; Gutierrez, L.M.; Perez-Mateo, M.; Closa, D. Pancreatic and pulmonary mast cells activation during experimental acute pancreatitis. World J. Gastroenterol. 2010, 16, 3411–3417. [Google Scholar] [CrossRef] [Green Version]
- Yonetci, N.; Oruc, N.; Ozutemiz, A.O.; Celik, H.A.; Yuce, G. Effects of mast-cell stabilization in cerulein-induced acute pancreatitis in rats. Int. J. Pancreatol. 2001, 29, 163–171. [Google Scholar] [CrossRef]
- Werner, J.; Dragotakes, S.C.; Fernandez-del Castillo, C.; Rivera, J.A.; Ou, J.; Rattner, D.W.; Fischman, A.J.; Warshaw, A.L. Technetium-99m-labeled white blood cells: A new method to define the local and systemic role of leukocytes in acute experimental pancreatitis. Ann. Surg. 1998, 227, 86–94. [Google Scholar] [CrossRef]
- Ayala, A.; Munoz, M.F.; Arguelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Grotto, D.; Maria, L.S.; Valentini, J.; Paniz, C.; Schmitt, G.; Garcia, S.C.; Pomblum, V.J.; Rocha, J.B.T.; Farina, M. Importance of the lipid peroxidation biomarkers and methodological aspects FOR malondialdehyde quantification. Química Nova 2009, 32, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.H.; Lim, J.W.; Namkung, W.; Kim, H.; Kim, K.H. Suppression of cerulein-induced cytokine expression by antioxidants in pancreatic acinar cells. Lab. Invest. 2002, 82, 1359–1368. [Google Scholar] [CrossRef] [Green Version]
- Kryzhevskii, V.V.; Nichitailo, M.E.; Medvetskii, E.B.; Moshkovskii, G. [The role of cytokines in pathogenesis of acute pancreatitis]. Klin. Khir 2000, 1, 54–57. [Google Scholar]
- Yu, J.H.; Kim, H. Oxidative stress and inflammatory signaling in cerulein pancreatitis. World J. Gastroenterol. 2014, 20, 17324–17329. [Google Scholar] [CrossRef] [PubMed]
- Akyuz, C.; Sehirli, A.O.; Topaloglu, U.; Ogunc, A.V.; Cetinel, S.; Sener, G. Protective Effects of Proanthocyanidin on Cerulein-induced Acute Pancreatic Inflammation in Rats. Gastroenterol. Res. 2009, 2, 20–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasari, L.P.; Khurana, A.; Anchi, P.; Aslam Saifi, M.; Annaldas, S.; Godugu, C. Visnagin attenuates acute pancreatitis via Nrf2/NFkappaB pathway and abrogates associated multiple organ dysfunction. Biomed. Pharmacother. 2019, 112, 108629. [Google Scholar] [CrossRef]
- Fu, Q.; Zhai, Z.; Wang, Y.; Xu, L.; Jia, P.; Xia, P.; Liu, C.; Zhang, X.; Qin, T.; Zhang, H. NLRP3 Deficiency Alleviates Severe Acute Pancreatitis and Pancreatitis-Associated Lung Injury in a Mouse Model. Biomed. Res. Int. 2018, 2018, 1294951. [Google Scholar] [CrossRef]
- Yu, J.; Ni, L.; Zhang, X.; Zhang, J.; Abdel-Razek, O.; Wang, G. Surfactant Protein D Dampens Lung Injury by Suppressing NLRP3 Inflammasome Activation and NF-kappaB Signaling in Acute Pancreatitis. Shock 2019, 51, 557–568. [Google Scholar] [CrossRef]
- Jiang, H.; Gong, T.; Zhou, R. The strategies of targeting the NLRP3 inflammasome to treat inflammatory diseases. Adv. Immunol. 2020, 145, 55–93. [Google Scholar] [CrossRef]
- Mangan, M.S.J.; Olhava, E.J.; Roush, W.R.; Seidel, H.M.; Glick, G.D.; Latz, E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discov. 2018, 17, 688. [Google Scholar] [CrossRef] [Green Version]
- Ozaki, E.; Campbell, M.; Doyle, S.L. Targeting the NLRP3 inflammasome in chronic inflammatory diseases: Current perspectives. J. Inflamm. Res. 2015, 8, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Lu, A.; Magupalli, V.G.; Ruan, J.; Yin, Q.; Atianand, M.K.; Vos, M.R.; Schroder, G.F.; Fitzgerald, K.A.; Wu, H.; Egelman, E.H. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 2014, 156, 1193–1206. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhang, S.; Xiao, Y.; Zhang, W.; Wu, S.; Qin, T.; Yue, Y.; Qian, W.; Li, L. NLRP3 Inflammasome and Inflammatory Diseases. Oxid. Med. Cell. Longev. 2020, 2020, 4063562. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.C. Host inflammatory responses to intracellular invaders: Review study. Life Sci. 2020, 240, 117084. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordaro, M.; Fusco, R.; D’Amico, R.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; Crupi, R.; Mandalari, G.; Cuzzocrea, S.; et al. Cashew (Anacardium occidentale L.) Nuts Modulate the Nrf2 and NLRP3 Pathways in Pancreas and Lung after Induction of Acute Pancreatitis by Cerulein. Antioxidants 2020, 9, 992. https://doi.org/10.3390/antiox9100992
Cordaro M, Fusco R, D’Amico R, Siracusa R, Peritore AF, Gugliandolo E, Genovese T, Crupi R, Mandalari G, Cuzzocrea S, et al. Cashew (Anacardium occidentale L.) Nuts Modulate the Nrf2 and NLRP3 Pathways in Pancreas and Lung after Induction of Acute Pancreatitis by Cerulein. Antioxidants. 2020; 9(10):992. https://doi.org/10.3390/antiox9100992
Chicago/Turabian StyleCordaro, Marika, Roberta Fusco, Ramona D’Amico, Rosalba Siracusa, Alessio Filippo Peritore, Enrico Gugliandolo, Tiziana Genovese, Rosalia Crupi, Giuseppina Mandalari, Salvatore Cuzzocrea, and et al. 2020. "Cashew (Anacardium occidentale L.) Nuts Modulate the Nrf2 and NLRP3 Pathways in Pancreas and Lung after Induction of Acute Pancreatitis by Cerulein" Antioxidants 9, no. 10: 992. https://doi.org/10.3390/antiox9100992